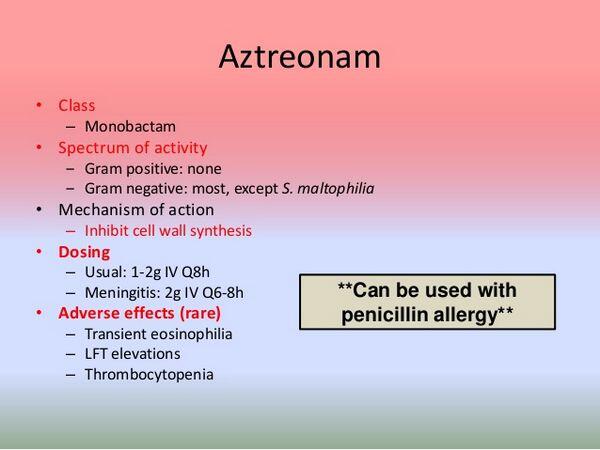
Aztreonam is a synthetic monocyclic b-lactam (i.e., monobactam) antibiotic.
Uses
Aztreonam is used for the treatment of complicated and uncomplicated urinary tract infections (including pyelonephritis and cystitis), lower respiratory tract infections (including pneumonia and bronchitis), septicemia, skin and skin structure infections (including those associated with postoperative wounds or ulcers and burns), intra-abdominal infections (including peritonitis), and gynecologic infections (including endometritis and pelvic cellulitis) caused by susceptible gram-negative aerobic bacteria.
Because aztreonam has a limited spectrum of activity and is active only against certain aerobic gram-negative bacteria, colonization or superinfection with aztreonam-resistant organisms may occur. (See Cautions: Precautions and Contraindications.)
The drug should not be used alone for empiric therapy in seriously ill patients if there is a possibility that the infection may be caused by gram-positive bacteria or if a mixed aerobic-anaerobic bacterial infection is suspected. In such infections, another anti-infective agent effective against the suspected, potentially aztreonam-resistant organism should initially be used concomitantly.
Aztreonam has been used safely and effectively in conjunction with an aminoglycoside, clindamycin, erythromycin, metronidazole, a penicillin, or vancomycin. Anti-infective agents that are potent inducers of b-lactamase production (e.g., cefoxitin, imipenem) should not be used concomitantly with aztreonam since the drugs may antagonize the antibacterial activity of aztreonam.
If an aminoglycoside is used concomitantly with aztreonam, renal function should be monitored, especially if high aminoglycoside dosage is used or if concomitant therapy is prolonged.
Prior to initiation of aztreonam therapy, appropriate specimens should be obtained for identification of the causative organism(s) and in vitro susceptibility tests. Aztreonam therapy may be started pending results of susceptibility tests, but should be discontinued and other appropriate anti-infective therapy substituted if the organism is found to be resistant to aztreonam.
Urinary Tract Infections
Aztreonam is used alone in adults for the treatment of complicated or uncomplicated urinary tract infections (UTIs), including pyelonephritis and initial and recurrent cystitis, caused by susceptible Enterobacter cloacae, Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, or Pseudomonas aeruginosa. The drug also has been effective when used alone in a limited number of adults for the treatment of UTIs caused by susceptible Citrobacter, K. oxytoca, Serratia marcescens, E. aerogenes, Morganella morganii, P. vulgaris, or Providencia.
Aztreonam has been effective in the treatment of cystitis or pyelonephritis caused by gram-negative aerobic bacteria resistant to aminopenicillins, first or second generation cephalosporins, and/or aminoglycosides. In controlled studies in men and women with UTIs, 5-14 days of aztreonam therapy was at least as effective as 5-14 days of therapy with an aminoglycoside (i.e., gentamicin, tobramycin, netilmicin [no longer commercially available in the US]) or a parenteral cephalosporin (i.e., cefamandole, cefotaxime, ceftriaxone).
Although aztreonam therapy generally is associated with less toxicity than aminoglycoside therapy, colonization or superinfection with gram-positive bacteria (especially Enterococcus faecalis [formerly Streptococcus faecalis]) has been reported more frequently with aztreonam therapy than with aminoglycoside therapy. In a controlled study in women with uncomplicated cystitis caused by E. coli, a single 1-g IM dose of aztreonam was as effective as 10 days of therapy with oral amoxicillin (250 mg 3 times daily); however, efficacy of a single dose of aztreonam in the treatment of these infections has not been established. Aztreonam was effective when used alone in a limited number of patients for the treatment of prostatitis caused by susceptible gram-negative bacteria; however, further study is needed to evaluate efficacy of the drug in prostatic infections. Aztreonam also has been effective when used alone in a limited number of children for the treatment of UTIs, including pyelonephritis and cystitis, caused by susceptible Enterobacteriaceae or Ps. aeruginosa.

Respiratory Tract Infections
Aztreonam is used for the treatment of lower respiratory tract infections, including pneumonia, bronchitis, or lung abscess, caused by susceptible gram-negative bacteria such as Enterobacter, E. coli, Haemophilus influenzae, K. pneumoniae, P. mirabilis, or Ps. aeruginosa. The drug also has been effective for the treatment of lower respiratory tract infections caused by susceptible S. marcescens, Citrobacter, Hafnia, K. oxytoca, Morganella, P. vulgaris, P. stuartii, or Moraxella catarrhalis.
Clinical improvement has been observed in some patients when aztreonam was used either alone or in conjunction with an aminoglycoside for the treatment of acute exacerbations of bronchopulmonary Ps. aeruginosa infections in patients with cystic fibrosis.
As with other anti-infective agents, a bacteriologic cure is rarely obtained and should not be expected in these patients. Because lower respiratory tract infections frequently are caused by gram-positive and/or anaerobic bacteria, an anti-infective agent active against these organisms, aztreonam should not be used alone for the empiric treatment of these infections.
A combination regimen of clindamycin and aztreonam has been used for initial empiric treatment of lower respiratory tract infections (especially nosocomial infections). The American Thoracic Society (ATS) has suggested aztreonam and clindamycin as an alternative regimen for empiric treatment of nosocomial pneumonia in patients hypersensitive to b-lactam anti-infectives (see Cautions: Precautions and Contraindications) and also has recommended a combination regimen of aztreonam and an aminoglycoside as one of several possible empiric regimens for patients with nosocomial pneumonia when Ps. aeruginosa or Acinetobacter may be involved (vancomycin should be added if methicillin-resistant staphylococci are suspected). In addition, for empiric therapy of community-acquired pneumonia (CAP) in patients at risk for Ps. aeruginosa infection who are hypersensitive to b-lactam anti-infectives, the ATS has suggested use of a regimen of aztreonam, an aminoglycoside, and an IV fluoroquinolone active against Streptococcus pneumoniae.
Septicemia
Aztreonam is used for the treatment of septicemia caused by susceptible Enterobacter, E. coli, K. pneumoniae, or Ps. aeruginosa and also has been effective when used in a limited number of adults for the treatment of septicemia caused by susceptible P. mirabilis, S. marcescens, Citrobacter, or H. influenzae. Aztreonam has generally been effective in either community-acquired or nosocomial septicemia known to be caused by susceptible gram-negative aerobes, and has been as effective as gentamicin or ceftazidime in the treatment of these infections.
Skin and Skin Structure Infections
Aztreonam is used as an adjunct to surgery in the management of abscesses, cutaneous infections, infections complicating hollow viscus perforations, or infections of serous surfaces caused by susceptible gram-negative aerobic bacteria. Aztreonam generally has been effective when used in adults for the treatment of skin and skin structure infections, including those associated with postoperative wounds or ulcers and burns, caused by susceptible Citrobacter, Enterobacter, E. coli, K. pneumoniae, P. mirabilis, Ps. aeruginosa, or S. marcescens.
Intra-abdominal and Gynecologic Infections
Aztreonam is used for the treatment of intra-abdominal infections, including peritonitis, caused by susceptible Citrobacter, Enterobacter, E. coli, Klebsiella, Ps. aeruginosa, or Serratia. The drug generally has been effective for the treatment of gynecologic infections, including endometritis or pelvic cellulitis, caused by susceptible Enterobacter, E. coli, Klebsiella, or P. mirabilis.
Because intra-abdominal and gynecologic infections generally are polymicrobial and frequently are mixed aerobic-anaerobic bacterial infections, aztreonam should not be used alone for the empiric treatment of these infections.
Clindamycin or metronidazole generally is used concomitantly with aztreonam for the initial treatment of intra-abdominal or gynecologic infections. In controlled studies in patients with intra-abdominal infections, including peritonitis, or with endometritis, aztreonam used in conjunction with clindamycin was as effective as gentamicin or tobramycin used in conjunction with clindamycin for the treatment of these infections.
In a limited number of patients, aztreonam used in conjunction with clindamycin was effective in the treatment of acute pelvic inflammatory disease (PID), although further study is needed to evaluate efficacy of the drug in the treatment of these infections.
Bone and Joint Infections
Aztreonam has been effective when used in a limited number of adults for the treatment of bone and joint infections, including osteomyelitis or septic arthritis, caused by susceptible Enterobacter, E. coli, Klebsiella, P. mirabilis, Ps. aeruginosa, or S. marcescens. The drug also has been effective when used in a limited number of children for the treatment of osteomyelitis, osteochondritis, or septic arthritis caused by susceptible Ps. aeruginosa or H. influenzae. An antistaphylococcal anti-infective agent (e.g., a penicillinase-resistant penicillin, vancomycin) should be used concomitantly with aztreonam if a gram-positive organism is known or suspected to also be present.
Gonorrhea and Associated Infections
A single 1-g IM dose of aztreonam has been effective for the treatment of uncomplicated urethral, endocervical, and/or anorectal gonorrhea caused by penicillinase- or nonpenicillinase-producing N. gonorrhoeae. However, aztreonam is not included in current CDC recommendations for the treatment of uncomplicated gonorrhea. Aztreonam generally has been ineffective for the treatment of pharyngeal gonococcal infections. For information on current recommendations regarding the treatment of gonorrhea and associated infections, see Uses: Gonorrhea and Associated Infections in Ceftriaxone 8:12.06..
Empiric Therapy in Febrile Neutropenic Patients
Aztreonam has been used in conjunction with vancomycin (with or without amikacin) for empiric anti-infective therapy in febrile granulocytopenic adults.
Because gram-positive bacteria (especially Staphylococcus epidermidis) are being reported with increasing frequency in febrile granulocytopenic patients and because aztreonam is inactive against these organisms, an anti-infective agent active against staphylococci (e.g., vancomycin) should be used in conjunction with aztreonam if the drug is used for empiric therapy in these patients.
Some clinicians suggest that a regimen of aztreonam and vancomycin can be used as an alternative empiric regimen in patients hypersensitive to penicillins and cephalosporins (see Cautions: Precautions and Contraindications). Published protocols for the treatment of infections in febrile neutropenic patients should be consulted for specific recommendations regarding selection of the initial empiric regimen, when to change the initial regimen, possible subsequent regimens, and duration of therapy in these patients.
Dosage and Administration
Reconstitution and Administration
Aztreonam is administered by IV injection or infusion or by deep IM injection.
The drug should be given IV rather than IM in patients with septicemia, localized parenchymal abscess (such as intra-abdominal abscess), peritonitis, or other severe systemic or life-threatening infection and when individual doses greater than 1 g are to be administered.
Aztreonam has been administered intraperitoneally in dialysis fluid. Vials or infusion bottles containing aztreonam should be shaken immediately and vigorously following addition of the appropriate diluent.
Reconstituted solutions of the drug should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Any unused solution should be discarded. When aztreonam is given IV via a common administration tubing used to administer another drug, especially one that is incompatible with aztreonam, the tubing should be flushed before and after aztreonam administration with an IV infusion solution compatible with both drugs; the drugs should not be given simultaneously.
When a Y-type IV administration set is used to administer aztreonam, careful attention should be given to the calculated volume of aztreonam solution to ensure that the entire dose is infused.
Intermittent IV Injection
For direct intermittent IV injection, the contents of a vial labeled as containing 500 mg, 1 g, or 2 g of aztreonam should be reconstituted by adding 6-10 mL of sterile water for injection. The appropriate dose of reconstituted solution may then be injected slowly over a period of 3-5 minutes either directly into a vein or into the tubing of a compatible IV solution.
ntermittent IV Infusion
For intermittent IV infusion, a 100-mL bottle labeled as containing 500 mg, 1 g, or 2 g of aztreonam may be reconstituted with a compatible IV infusion solution to provide a solution with a final concentration not exceeding 20 mg/mL; each g of aztreonam should be reconstituted with at least 50 mL of compatible IV infusion solution.
Alternatively, a vial labeled as containing 500 mg, 1 g, or 2 g of aztreonam may be initially reconstituted using at least 3 mL of sterile water for injection per g of drug and then diluted further by adding the reconstituted solution to a compatible IV infusion solution to provide a solution with a final concentration not exceeding 20 mg/mL.
A volume control IV administration set may be used to add the appropriate dose of the initially reconstituted aztreonam solution to the compatible IV infusion solution during administration; this final dilution should provide a solution with a concentration of 20 mg/mL or less.
Thawed solutions of the commercially available frozen aztreonam injection in dextrose should be administered only by intermittent IV infusion. The commercially available frozen aztreonam injections should not be thawed by warming them in a water bath or by exposure to microwave radiation.
A precipitate may form while the commercially available frozen injection in dextrose is frozen; however, this usually will dissolve with little or no agitation upon reaching room temperature. After thawing at room temperature, the containers should be checked for minute leaks by firmly squeezing the bag.
The injection should be discarded if the container seal or outlet ports are not intact or leaks are found or if the solution is cloudy, discolored, or contains a precipitate. Additives should not be introduced into the injection container or be infused simultaneously through the same IV line.
The injections should not be used in series connections with other plastic containers, since such use could result in air embolism from residual air being drawn from the primary container before administration of fluid from the secondary container is complete. The manufacturer recommends that the IV administration set be replaced every 48 hours. Intermittent IV infusions of aztreonam should be infused over 20-60 minutes.
IM Injection
For IM administration, a vial labeled as containing 500 mg, 1 g, or 2 g of aztreonam may be reconstituted with sterile water for injection, 0.9% sodium chloride injection, bacteriostatic water for injection (with benzyl alcohol or parabens), or bacteriostatic sodium chloride injection (with benzyl alcohol). The vials should be reconstituted by adding at least 3 mL of one of these diluents per g of aztreonam.
IM injections of aztreonam should be made deeply into a large muscle, such as the upper outer quadrant of the gluteus maximus or lateral part of the thigh, using usual techniques and precautions. Aztreonam generally is well tolerated when given IM and should not be admixed with local anesthetic agents.
Dosage
Dosage and route of administration of aztreonam should be determined by the type and severity of infection, susceptibility of the causative organism, and the condition of the patient. Dosages lower than the usual recommended dosage of the drug should not be used.
Adult Dosage
The usual adult IV or IM dosage of aztreonam for the treatment of urinary tract infections is 500 mg or 1 g every 8 or 12 hours. For the treatment of moderately severe systemic infections, adults should receive 1 g IV or IM or 2 g IV every 8 or 12 hours. Severe systemic or life-threatening infections in adults, especially infections caused by Pseudomonas aeruginosa, may require 2 g IV every 6 or 8 hours.
The maximum adult dosage of aztreonam recommended by the manufacturer is 8 g daily. In geriatric patients, renal function should be used as the major determinant of dosage since these patients may have renal impairment. (See Dosage: Dosage in Renal and Hepatic Impairment.)
Pediatric Dosage
The usual dosage of aztreonam for pediatric patients 9 months of age or older with normal renal function is 30 mg/kg IV every 8 hours for the treatment of mild to moderate infections or 30 mg/kg IV every 6 or 8 hours for the treatment of moderate to severe infections. The maximum recommended dosage of aztreonam in pediatric patients is 120 mg/kg daily; however, higher dosage may be warranted in those with cystic fibrosis.
An IV dosage of 50 mg/kg every 6 or 8 hours (i.e., 150-200 mg/kg daily) has been suggested for the treatment of infections in children with cystic fibrosis. Although safe use of aztreonam in neonates has not been established, the American Academy of Pediatrics (AAP) recommends that neonates younger than 1 week of age receive 30 mg/kg of aztreonam every 12 hours (for those weighing 2 kg or less) or every 8 hours (for those weighing more than 2 kg) and that neonates 1-4 weeks of age receive 30 mg/kg every 8 hours (for those weighing 2 kg or less) or every 6 hours (for those weighing more than 2 kg). The AAP suggests that a dosage of 30 mg/kg every 12 hours is appropriate for very-low-birthweight neonates (i.e., less than 1.2 kg).
Duration of Therapy
The duration of aztreonam therapy depends on the type and severity of infection and should be determined by the clinical and bacteriologic response of the patient. For most infections, therapy should be continued for at least 48 hours after the patient becomes asymptomatic or evidence of eradication of the infection has been obtained. Persistent infections may require several weeks of aztreonam therapy.
The usual duration of aztreonam therapy for the treatment of uncomplicated urinary tract infections is 5-10 days; therapy should be continued for at least 10-18 days for the treatment of complicated urinary tract infections. Although some lower respiratory tract infections have been treated effectively with 5-18 days of aztreonam therapy, severe infections (including septicemia) generally require more prolonged therapy.
Dosage in Renal and Hepatic Impairment
Renal Impairment
In patients with renal impairment, doses and/or frequency of administration of aztreonam should be modified in response to the degree of renal impairment. Data are insufficient to date to make dosage recommendations for pediatric patients with impaired renal function.
Serum creatinine concentrations alone may not be sufficiently accurate to assess the degree of renal impairment, especially in geriatric adults; dosage of aztreonam preferably should be based on the patient’s measured or estimated creatinine clearance. The patient’s creatinine clearance (Ccr) can be estimated by using the following formulas: Adults with creatinine clearances greater than 30 mL/minute per 1.73 m may receive the usual adult dosage of aztreonam.
Adults with creatinine clearances of 10-30 mL/minute per 1.73 m should receive an initial 1- or 2-g loading dose of aztreonam followed by maintenance doses equal to one-half the usual dose (i.e., 250 mg, 500 mg, or 1 g) given at the usual dosage intervals. In adults with creatinine clearances less than 10 mL/minute per 1.73 m (including hemodialysis patients), an initial loading dose equal to the usual dose (i.e., 500 mg, 1 g, or 2 g) should be used followed by maintenance doses equal to one-fourth the usual dose (i.e., 125 mg, 250 mg, or 500 mg) given at the usual dosage intervals. Because aztreonam is removed by hemodialysis, patients undergoing hemodialysis should receive a supplemental aztreonam dose equal to one-eighth the initial dose (i.e., 62. mg, 125 mg, or 250 mg) immediately after each dialysis period.
Adults undergoing continuous ambulatory peritoneal dialysis (CAPD) who have systemic infections should receive an initial loading dose of aztreonam equal to the usual dose (i.e., 500 mg, 1 g, or 2 g) followed by maintenance doses equal to one-fourth the usual dose (i.e., 125 mg, 250 mg, or 500 mg) given at the usual dosage intervals. It has been suggested that adults undergoing CAPD who have peritonitis caused by susceptible organisms may receive a 1-g loading dose of aztreonam given IV followed by maintenance doses of 500 mg given intraperitoneally in 2 L of dialysate every 6 hours.
Hepatic Impairment
Experience with aztreonam in patients with impaired hepatic function is limited. Although some clinicians recommend that aztreonam dosage be decreased by 20-25% in patients with alcoholic cirrhosis, especially if long-term therapy with the drug is required, other clinicians suggest that this decrease in dosage is unnecessary unless renal function also is impaired. Modification of usual dosage probably is unnecessary in patients with stable primary biliary cirrhosis or other chronic hepatic disease unless renal function also is impaired.
Cautions
Adverse effects reported with aztreonam are similar to those reported with other b-lactam antibiotics, and the drug generally is well tolerated. Adverse effects have been reported in 7% or less of patients receiving aztreonam and have required discontinuance in about 2% of patients.
GI Effects
Diarrhea, nausea, and vomiting have been reported in about 1-2% and GI bleeding, abdominal cramps, and bloating have been reported in less than 1% of patients receiving aztreonam. A transient, unusual taste has been reported occasionally during and after IV infusion of aztreonam, and numbness of the tongue, oral ulceration, and halitosis have been reported in less than 1% of patients receiving the drug.
Although animal data suggest that the potential for aztreonam to produce colitis is minimal, Clostridium difficile-associated diarrhea and colitis (also known as antibiotic-associated pseudomembranous colitis), caused by toxin-producing clostridia resistant to aztreonam, has occurred in less than 1% of patients during or following discontinuance of aztreonam therapy. C. difficile-associated diarrhea and colitis may range in severity from mild to life-threatening. Mild cases of C. difficile-associated colitis may respond to discontinuance of the drug alone, but diagnosis and management of moderate to severe cases should include appropriate bacteriologic studies and treatment with fluid, electrolyte, and protein supplementation as indicated; rarely, cautious use of sigmoidoscopy (or other appropriate endoscopic examination) may be considered necessary.
If colitis is moderate to severe or is not relieved by discontinuance of aztreonam, appropriate anti-infective therapy (e.g., oral metronidazole or vancomycin) should be administered. Isolation of the patient may be advisable.
Other causes of colitis also should be considered. Aztreonam therapy exerts a selective effect on normal bowel flora. Total bacterial counts of normal fecal aerobic gram-negative bacteria are decreased, but total counts of normal fecal anaerobic bacteria generally are unaffected during or following therapy with oral or IV aztreonam. An increase in total fecal counts of enterococci has occurred in healthy adults receiving oral or IV aztreonam, but total fecal counts of staphylococci and fungi generally are unaffected.
Bacterial counts of normal fecal flora generally return to pretreatment levels within 1 week after aztreonam therapy is discontinued.
Dermatologic and Sensitivity Reactions
Rash, with or without eosinophilia, has been reported in about 1-2% of patients receiving aztreonam. Rash generally has been mild, transient, pruritic, and erythematous, but rarely has been maculopapular or urticarial.
Pruritus, purpura, erythema multiforme, toxic epidermal necrolysis, urticaria, exfoliative dermatitis, petechiae, and diaphoresis have been reported in less than 1% of patients receiving the drug. Toxic epidermal necrolysis has been reported in association with aztreonam in a few patients undergoing bone marrow transplant; these patients had multiple risk factors including graft versus host disease, sepsis, and radiation therapy, and were receiving other drugs that have been associated with toxic epidermal necrolysis. Immediate hypersensitivity reactions, including anaphylaxis, bronchospasm, generalized urticaria with or without palpebral and lingual edema and respiratory impairment, and a severe episode of shock, rash, and eosinophilia, have been reported in less than 1% of patients receiving aztreonam.
The immunogenic risk of aztreonam has not been fully determined. When aztreonam was administered to individuals with a history of hypersensitivity to penicillins and/or cephalosporins in one study, less than 1% of such individuals had a possible hypersensitivity reaction to aztreonam (i.e., an urticarial rash).
Similarly, when single IM doses of aztreonam were given to individuals with a history of positive skin test reactions to penicillin reagents and negative skin test reactions to aztreonam in another study, there were no immediate hypersensitivity reactions to aztreonam, but a localized rash compatible with a fixed drug eruption occurred in one individual. Urticaria and pharyngeal edema also have occurred when aztreonam was used in a patient with a history of penicillin hypersensitivity.
Studies in rabbits and humans suggest that antibodies produced in response to penicillin G (including IgE antibodies to major and minor determinants) or to cephalothin (no longer commercially available in the US) show negligible cross-reactivity with aztreonam. Likewise, antibodies produced in response to aztreonam have had negligible cross-reactivity with penicillin G, cephalothin, and cefotaxime. In one study in healthy men who had not previously received aztreonam, no IgE antibody response to aztreonam was detectable after therapy with the drug (500-mg or 1-g doses every 8 hours for 7 days). A few of these individuals did have naturally occurring side-chain-specific IgG antibodies to aztreonam, but only one demonstrated an IgG response to the drug.
Although results of this study and some rabbit studies suggest that aztreonam may be only weakly immunogenic compared with penicillin G, studies in patients who have received multiple courses of aztreonam therapy and further studies to identify aztreonam degradation products and to evaluate their immunogenic potential are necessary to more fully assess the immunogenicity of the drug.
Although there appears to be little cross allergenicity between aztreonam and bicyclic b-lactam antibiotics, the possibility that cross-sensitivity to aztreonam may occur in patients with a history of hypersensitivity to other b-lactam antibiotics should be considered. (See Cautions: Precautions and Contraindications.) If a hypersensitivity reaction to aztreonam occurs, the drug should be discontinued and appropriate supportive treatment initiated (e.g., vasopressors, antihistamines, corticosteroids, maintenance of ventilation). Serious hypersensitivity reactions may require epinephrine and other emergency measures.
Hematologic Effects
Transient eosinophilia has been reported in up to 11% and leukopenia, neutropenia, thrombocytopenia, pancytopenia, anemia, leukocytosis, and thrombocytosis have been reported in less than 1% of patients receiving aztreonam.
Decreased hemoglobin concentration and hematocrit have been reported rarely. Positive direct antiglobulin (Coombs’) test results, without clinical or laboratory evidence of hemolytic anemia, also have been reported rarely. Slight prolongation of the prothrombin time (up to 1.5 times baseline values) and activated partial thromboplastin time, without evidence of bleeding, has been reported rarely during aztreonam therapy.
Clinically apparent bleeding, possibly related to aztreonam therapy, has been reported in several seriously ill patients who received aztreonam as well as several other drugs. In vitro studies indicate that high concentrations of aztreonam (i.e., greater than 2.7 mg/mL) can inhibit adenosine diphosphate (ADP)-, collagen-, and epinephrine-induced platelet aggregation. Although aztreonam therapy (2 g IV every 6 hours) may cause slight inhibition of ADP-induced platelet aggregation, usual dosages of the drug do not appear to have any clinically important effects on platelet function or blood coagulation in adults with normal renal and hepatic function.
Hepatic Effects
Transient increases in serum AST (SGOT), ALT (SGPT), and alkaline phosphatase concentrations have been reported in 2-40% of patients receiving aztreonam. In most reported cases, liver enzyme concentrations were increased only up to 3 times normal, were not associated with symptoms of hepatobiliary dysfunction, and returned to pretreatment concentrations shortly after aztreonam therapy was completed. Rarely, serum AST and ALT concentrations have increased to more than 10 times normal during aztreonam therapy; hepatitis and jaundice and other manifestations of hepatotoxicity have been reported in less than 1% of patients receiving the drug. Transient increases in serum bilirubin, LDH, and Gamma-glutamyltransferase (γ-glutamyl transpeptidase, GGT, GGTP) have been reported rarely.
Local Effects
Phlebitis and/or thrombophlebitis have been reported in 2-3% of patients receiving aztreonam IV. Phlebitis and thrombophlebitis are usually mild, occur about 1 week after initiation of aztreonam therapy, and generally are relieved by changing administration sites, applying warm packs, and other general measures. Discomfort, pain, and swelling at the injection site have been reported in up to about 3% of patients receiving aztreonam IM, but the drug generally is well tolerated when administered by this route and should not be admixed with local anesthetic agents.
Cardiovascular Effects
Hypotension and transient ECG changes, including ventricular bigeminy and ventricular premature complexes (VPCs, PVCs), have been reported in less than 1% of patients receiving aztreonam. Bradycardia, flushing, chest pain, lower limb edema, and subclavian vein thrombosis have occurred rarely.
Nervous System Effects
Seizures, confusion, disturbed mental processes, insomnia, dizziness, vertigo, paresthesia, weakness, fatigue, malaise, and headache have occurred in less than 1% of patients receiving aztreonam.
Renal Effects
Transient increases in BUN and/or serum creatinine concentrations have been reported rarely in patients receiving aztreonam. Aztreonam does not appear to be nephrotoxic in humans. In at least one patient, acute renal failure associated with rash and eosinophilia occurred within about 9 days after initiating aztreonam therapy. Urinary concentrations of b2-microglobulin and excretion of thermophilic aminopeptidase (alanine aminopeptidase) and N-acetyl-B-glucosaminidase, enzymes that originate from renal proximal tubular cells, generally are unaffected in healthy adults receiving usual dosages of the drug. In addition, urinary excretion of N-acetyl-B-glucosaminidase reportedly is unaffected during aztreonam therapy in patients with urinary tract infections and impaired renal function.
Other Adverse Effects
Fever, chills, cold sweats, dyspnea, sneezing, nasal congestion, tinnitus, impaired hearing in one ear, diplopia, myalgia, vaginitis, vaginal candidiasis, and breast tenderness have been reported in less than 1% of patients receiving aztreonam.
Precautions and Contraindications
Prior to initiation of aztreonam therapy, careful inquiry should be made concerning previous hypersensitivity reactions to anti-infective agents, including other b-lactam antibiotics, or to other drugs. There is clinical and laboratory evidence of partial cross-allergenicity among bicyclic b-lactam antibiotics including penicillins, cephalosporins, cephamycins, and carbapenems.
Although there appears to be little cross-allergenicity between aztreonam and bicyclic b-lactam antibiotics, hypersensitivity reactions to aztreonam have occurred rarely when the drug was used in patients with a history of hypersensitivity to penicillins and/or cephalosporins.(See Cautions: Dermatologic and Sensitivity Reactions.) Individuals with a history of immediate type I hypersensitivity reactions (e.g., IgE-mediated urticaria, anaphylaxis) to penicillins and/or cephalosporins should be monitored closely during aztreonam therapy.
Although it has not been proven that allergic reactions to antibiotics are more frequent in atopic individuals, the manufacturer states that aztreonam should be used with caution in individuals with a history of allergy, particularly to drugs. As with other anti-infective agents, use of aztreonam may result in overgrowth of nonsusceptible organisms, especially gram-positive bacteria (e.g., enterococci, Staphylococcus aureus, Streptococcus pneumoniae) or fungi.
Colonization or superinfection with aztreonam-resistant organisms has occurred in up to 60% of patients receiving the drug, and superinfections have required treatment with another anti-infective agent in about 4-11% of patients. Use of indwelling catheters or the presence of tracheotomy sites or open, draining wounds appears to contribute to the occurrence of superinfections during aztreonam therapy. Resistant strains of some organisms (e.g., Pseudomonas aeruginosa, Klebsiella pneumoniae) have developed during aztreonam therapy. (See Resistance.)
Careful monitoring of the patient is essential. If superinfection occurs, appropriate therapy should be instituted. Because Clostridium difficile-associated diarrhea and colitis has been reported with the use of anti-infective agents, including aztreonam, it should be considered in the differential diagnosis of patients who develop diarrhea during or following therapy with the drug.
When aztreonam is used in patients with impaired renal or hepatic function, appropriate laboratory tests should be monitored during therapy. Doses and/or frequency of administration of aztreonam should be decreased in patients with impaired renal function, since serum concentrations of the drug are higher and prolonged in these patients compared with patients with normal renal function.
Some clinicians suggest that liver function tests (i.e., serum hepatic enzyme concentrations) be determined once weekly in patients receiving aztreonam; however, the manufacturer and other clinicians question the necessity of this precaution. Aztreonam is contraindicated in individuals who are hypersensitive to the drug or any component in the formulation.
Pediatric Precautions
Safety and efficacy of aztreonam in children younger than 9 months of age have not been established. The manufacturer states that use of IV aztreonam in children 9 months of age or older is supported by evidence from adequate and well-controlled studies in adults and additional efficacy, safety, and pharmacokinetic data from noncomparative clinical studies in pediatric patients.
However, data are insufficient to date to determine safety and efficacy of IV aztreonam in younger children or for the treatment of septicemia or skin and skin structure infections suspected or known to be caused by Haemophilus influenzae type b. In addition, the manufacturer states that data are insufficient to date to evaluate IM administration of aztreonam in pediatric patients or use of the drug in pediatric patients with impaired renal function.
Aztreonam has been used IM or IV in a limited number of neonates and infants as young as 1 month of age without unusual adverse effects. In clinical studies evaluating aztreonam in pediatric patients, less than 1% have required discontinuance of the drug because of adverse effects. Rash, diarrhea, and fever have been reported in 1-4.3% of pediatric patients. In pediatric patients receiving IV aztreonam, pain was reported in 12% and erythema, induration, or phlebitis were reported in 0.9-2.9% of patients overall; in US patients, pain occurred in 1.5% and other local reactions occurred in about 0.5%.
Eosinophilia, neutropenia, or increased platelet count has been reported in 6.3, 3.2, or 3.6% of pediatric patients, respectively, and increased serum AST, ALT, or serum creatinine has been reported in 3.8-6.5%. In US pediatric studies, neutropenia (absolute neutrophil count less than 1000/mm3) occurred in 11.% of patients younger than 2 years of age receiving aztreonam in a dosage of 30 mg/kg every 6 hours and increased serum AST and ALT (greater than 3 times the upper limit of normal) occurred in 15-20% of patients 2 years of age or older receiving a dosage of 50 mg/kg every 6 hours. It is unclear whether the increased frequency of these adverse effects was related to increased severity of illness in the patients or aztreonam dosage administered.
Mutagenicity and Carcinogenicity
No evidence of mutagenicity was seen at the chromosomal or gene level when aztreonam was evaluated in several in vitro and in vivo test systems. The drug was not mutagenic in the Ames microbial mutagen test. At concentrations up to 20 mg/kg, aztreonam was inactive in the mouse lymphoma cell forward mutation assay, with and without metabolic activation. The drug did not cause chromosomal aberrations in bone marrow cells in mice receiving subcutaneous dosages of 0.4, 1.2, or 3.6 g/kg daily for 5 days. In addition, at concentrations up to 40 mg/mL, the drug did not demonstrate any potential to induce chromosomal aberrations in purified human lymphocytes, with and without metabolic activation. Studies have not been performed to date to evaluate the carcinogenic potential of aztreonam.
Pregnancy, Fertitlity and Lactation
Reproduction studies in rabbits and rats using daily aztreonam dosages up to 5 and 15 times the maximum recommended human dose, respectively, have not revealed evidence of embryotoxicity, fetotoxicity, or teratogenicity.
In rats who received aztreonam dosages 15 times the maximum recommended human dose during late gestation and lactation, there were no drug-induced changes in any maternal, fetal, or neonatal parameter monitored. However, in reproduction studies in 2 generations of rats receiving aztreonam in daily dosages up to 20 times the maximum recommended human dosage prior to and during gestation and lactation, there was a slightly reduced survival rate during the lactation period in the offspring of rats that received the highest dosage, but not in the offspring of rats that received 5 times the maximum recommended human dosage.
There are no adequate and controlled studies to date using aztreonam in pregnant women; it is also not known whether the drug can cause fetal harm or affect reproduction capacity when administered to pregnant women.
Aztreonam should be used during pregnancy only when clearly needed. Reproduction studies in male and female rats using aztreonam dosages up to 20 times the maximum recommended human daily dosage have not revealed evidence of impaired fertility. Because aztreonam is distributed into milk in low concentrations and because safety of aztreonam in neonates has not been fully evaluated to date, temporary discontinuance of nursing should be considered during therapy with the drug in lactating women.
Drug Interactions
Probenecid
Concomitant administration of probenecid slows the rate of renal tubular secretion of aztreonam. This effect, however, is not sufficient to be of therapeutic benefit since it produces only a 5% increase in serum aztreonam concentrations and an 11% increase in the serum half-life of the drug. Concomitant probenecid also appears to decrease the binding of aztreonam to plasma proteins by about 13%, presumably by competing with the drug for plasma and tissue protein binding sites, and to decrease the steady-state volume of distribution of aztreonam by about 16%.
Aminoglycosides
The antibacterial activity of aztreonam and aminoglycosides is additive or synergistic in vitro against most strains of Pseudomonas aeruginosa and some strains of Ps. cepacia, Ps. fluorescens, or Ps. maltophilia. The combination of aztreonam and an aminoglycoside also is synergistic against some Enterobacteriaceae, including some strains of Enterobacter, Escherichia coli, Klebsiella, or Serratia. In vitro, the combination of aztreonam and an aminoglycoside has occasionally been synergistic against Acinetobacter, although the combination more frequently is only additive or indifferent against this organism. The combination of aztreonam and an aminoglycoside generally is indifferent against gram-positive bacteria, including Staphylococcus aureus, S. epidermidis, or Enterococcus faecalis (formerly Streptococcus faecalis). In a study in healthy adults who received a single 1-g IV dose of aztreonam concomitantly with a single 80-mg IV dose of gentamicin, peak serum concentrations of aztreonam were decreased by about 13%, but other pharmacokinetic parameters of the drugs (e.g., half-lives, areas under the serum concentration-time curves) were not affected by concomitant administration. Further study using multiple doses of the drugs and/or higher doses is probably needed to confirm that there are no clinically important pharmacokinetic interactions between aztreonam and gentamicin.
B-Lactam Antibiotics
An additive or synergistic effect has occurred in vitro against some strains of Ps. aeruginosa when aztreonam was used concomitantly with piperacillin, cefoperazone, or cefotaxime. The combination of aztreonam and ampicillin, piperacillin, cefoperazone, or cefotaxime generally is indifferent or only slightly additive against Enterobacteriaceae, including Enterobacter, E. coli, S. marcescens, or Klebsiella. In vitro, the combination of aztreonam and cefoxitin has been synergistic against some strains of Enterobacter, E. coli, Klebsiella, S. marcescens, Salmonella, or Shigella. However, antagonism has occurred in vitro when aztreonam was used in combination with cefoxitin against Enterobacter or S. marcescens.
Antagonism also has occurred in vitro when imipenem was used in combination with aztreonam against Ps. aeruginosa. Antagonism between aztreonam and these anti-infectives may occur because cefoxitin and imipenem are potent b-lactamase inducers and can derepress inducible, chromosomally mediated b-lactamases in gram-negative bacteria that possess these enzymes (e.g., Enterobacter, Serratia, Ps. aeruginosa). Although aztreonam is relatively stable against hydrolysis by inducible b-lactamases, it has been suggested that the enzymes may inactivate aztreonam by binding to the drug and preventing access to penicillin-binding proteins. Because the combinations may be antagonistic, anti-infective agents that are potent inducers of b-lactamase production (e.g., cefoxitin, imipenem) should not be used concomitantly with aztreonam.
Other Anti-infectives
In vitro, the antibacterial activity of aztreonam and clindamycin has been synergistic against some strains of E. coli, Klebsiella, or Enterobacter, although the combination more frequently is indifferent or additive against these organisms. Indifferent or slightly additive effects also have been reported when aztreonam was used in conjunction with clindamycin or metronidazole against anaerobic bacteria. In a study in healthy adults who received a single IV dose of aztreonam concomitantly with a single IV dose of metronidazole or clindamycin, peak serum concentrations of aztreonam were decreased by about 10% in patients receiving concomitant metronidazole and total urinary excretion of aztreonam was increased by about 5% in those receiving clindamycin.
These changes in pharmacokinetic parameters did not appear to be of clinical importance and other parameters (e.g., half-lives, areas under the serum concentration-time curves) were not affected by concomitant administration of the drugs. Further study using multiple and/or higher doses of the drugs probably are needed to confirm that there are no clinically important pharmacokinetic interactions between aztreonam and metronidazole or clindamycin. Results of an in vitro study using Klebsiella pneumoniae indicate that chloramphenicol can antagonize the bactericidal activity of aztreonam.
It has been suggested that if concomitant use of the drugs is indicated, chloramphenicol should be administered a few hours after aztreonam; however, the necessity of this precaution has not been established.
Clavulanic Acid
In vitro studies indicate that the combination of aztreonam and clavulanic acid, a b-lactamase inhibitor, is synergistic against some strains of b-lactamase-producing Enterobacter, Klebsiella, or Bacteroides fragilis resistant to aztreonam alone.
The combination of aztreonam and clavulanic acid may also be antagonistic against some organisms. Clavulanic acid can induce production of chromosomally mediated b-lactamases in some gram-negative bacteria (e.g., Enterobacter, Ps. aeruginosa) and therefore could interfere with the antibacterial activity of aztreonam by a mechanism similar to that seen with cefoxitin or imipenem. Concomitant use of clavulanic acid and aztreonam does not alter the in vitro susceptibility of Staphylococcus aureus to aztreonam since resistance to the drug in these organisms is intrinsic.
Other Drugs
Furosemide can increase serum aztreonam concentrations, but such increases are clinically unimportant.
Laboratory Test Interferences
Tests for Urinary Glucose
Like most other currently available b-lactam antibiotics, aztreonam interferes with urinary glucose determinations using cupric sulfate (e.g., Benedict’s solution, Clinitest®), but does not appear to interfere with glucose oxidase tests (e.g., Diastix®, Tes-Tape®).
Tests for Creatinine
Aztreonam does not appear to interfere with serum or urinary creatinine determinations when the Jaffe reaction is used.
Acute Toxcicity
Limited information is available on the acute toxicity of aztreonam in humans. The IV LD50 of the drug is 3.3 g/kg in mice, and the intraperitoneal LD50 is 6.6 g/kg in rats. If acute overdosage of aztreonam occurs, hemodialysis and/or peritoneal dialysis may enhance elimination of the drug from the body.
Mechanism of Action
Like bicyclic b-lactam antibiotics, the antibacterial activity of aztreonam results from inhibition of mucopeptide synthesis in the bacterial cell wall. Aztreonam has a high affinity for and preferentially binds to penicillin-binding protein 3 (PBP 3) of susceptible gram-negative bacteria.
The drug also has some affinity for PBP 1a of these bacteria, but little or no affinity for PBPs 1b, 2, 4, 5, or 6. Because PBP 3 is involved in septation, aztreonam causes the formation of abnormally elongated or filamentous forms in susceptible gram-negative bacteria. As a consequence, cell division is inhibited and breakage of the cell wall occurs resulting in lysis and death.
Studies using Staphylococcus aureus indicate that aztreonam does not bind to the essential PBPs of gram-positive bacteria. Aztreonam also has poor affinity for the PBPs of anaerobic bacteria. The drug, therefore, generally is inactive against these organisms. Aztreonam usually is bactericidal in action. Since aztreonam has poor affinity for PBPs 1a and 1b of susceptible gram-negative bacteria, it is not as rapidly bactericidal as some other b-lactam antibiotics (e.g., imipenem, cefotaxime, cefoxitin, ceftriaxone) against these organisms.
For most susceptible Enterobacteriaceae, the minimum bactericidal concentration (MBC) of aztreonam is equal to or only 2-4 times higher than the minimum inhibitory concentration (MIC) of the drug. For Pseudomonas aeruginosa, the MBC of aztreonam is usually only 2 times higher than the MIC, but may be up to 125 times higher than the MIC for some strains of the organism. Spectrum Aztreonam has a narrow spectrum of activity. The drug is active in vitro against many gram-negative aerobic bacteria, including most Enterobacteriaceae and Pseudomonas aeruginosa, but has little or no activity against gram-positive aerobic bacteria or against anaerobic bacteria. Aztreonam is inactive against Chlamydia, Mycoplasma, fungi, and viruses.
In Vitro Susceptibility Testing
Results of in vitro susceptibility tests with aztreonam may be affected by the size of the inoculum. MICs of aztreonam for Enterobacteriaceae or Ps. aeruginosa are generally only 1-4 times greater when the size of the inoculum is increased from 103 or 104 to 106 colony-forming units (CFU) per mL; however, MICs for these organisms may be 15-500 times greater when the inoculum is increased from 104 or 105 to 107-108 CFU/mL. The clinical importance of this inoculum effect has not been determined. It has been suggested that it may occur because aztreonam causes the formation of abnormally elongated or filamentous forms in susceptible gram-negative bacteria, but is not rapidly bactericidal, which may result in an initial increase in bacterial mass. In macrobroth or microbroth dilution susceptibility tests that use turbidity to determine the presence of bacterial growth, this initial increase in bacterial mass could result in a corresponding increase in optical density that could be interpreted as growth or resistance.
The increase in bacterial mass may not result in visually detectable turbidity when an inoculum smaller than 105 CFU/mL is used. Although results of aztreonam susceptibility tests generally are unaffected by the presence of serum, the minimum bactericidal concentration (MBC) of the drug for Ps. aeruginosa or Proteus mirabilis is 2-128 times greater in the presence of 75% serum. MICs and MBCs of aztreonam generally are unaffected by the type of media or pH changes between 6-8. The drug is equally active in vitro when susceptibility tests are performed under aerobic or anaerobic conditions.
The National Committee for Clinical Laboratory Standards (NCCLS) states that, if results of in vitro susceptibility testing indicate that a clinical isolate is susceptible to aztreonam, then an infection caused by this strain may be appropriately treated with the dosage of the drug recommended for that type of infection and infecting species, unless otherwise contraindicated. If results indicate that a clinical isolate has intermediate susceptibility to aztreonam, then the strain has a minimum inhibitory concentration (MIC) that approaches usually attainable blood and tissue concentrations and response rates may be lower than for strains identified as susceptible.
Therefore, the intermediate category implies clinical applicability in body sites where the drug is physiologically concentrated (e.g., urine) or when a high dosage of the drug can be used. This intermediate category also includes a buffer zone which should prevent small, uncontrolled technical factors from causing major discrepancies in interpretation, especially for drugs with narrow pharmacotoxicity margins. If results of in vitro susceptibility testing indicate that a clinical isolate is resistant to aztreonam, the strain is not inhibited by systemic concentrations of the drug achievable with usual dosage schedules and/or MICs fall in the range where specific microbial resistance mechanisms are likely and efficacy has not been reliably demonstrated in clinical studies.
Disk Susceptibility Tests
When the disk-diffusion procedure is used to test susceptibility to aztreonam, a disk containing 30 mcg of the drug should be used. When disk-diffusion susceptibility testing is performed according to NCCLS standardized procedures using NCCLS interpretive criteria, Enterobacteriaceae, Pseudomonas aeruginosa, and Acinetobacter with growth inhibition zones of 22 mm or greater are susceptible to aztreonam, those with zones of 16-21 mm have intermediate susceptibility, and those with zones of 15 mm or less be are resistant to the drug. When disk-diffusion susceptibility testing is performed according to NCCLS standardized procedures using Haemophilus test medium (HTM), Haemophilus with growth inhibition zones of 26 mm or greater are considered susceptible to aztreonam. Because of limited data on resistant strains, NCCLS recommends than any Haemophilus isolate that appears to be nonsusceptible to aztreonam should be submitted to a reference laboratory for further testing.
Dilution Susceptibility Tests
When dilution susceptibility testing (agar or broth dilution) is performed according to NCCLS standardized procedures using NCCLS interpretive criteria, Enterobacteriaceae and Ps. aeruginosa and other non-Enterobacteriaceae gram-negative bacilli (e.g., other Pseudomonas spp., Acinetobacter, Stenotrophomonas maltophilia) with MICs of 8 mcg/mL or less are susceptible to aztreonam, those with MICs of 16 mcg/mL have intermediate susceptibility, and those with MICs of 32 mcg/mL or greater are resistant to the drug. When NCCLS standardized procedures for broth dilution are performed using HTM, Haemophilus with MICs of 2 mcg/mL or less are considered susceptible to aztreonam. Because of limited data on resistant strains, NCCLS recommends than any Haemophilus isolate that appears to be nonsusceptible to aztreonam be submitted to a reference laboratory for further testing.
Gram-positive Aerobic Bacteria
Aztreonam has little activity against most gram-positive aerobic bacteria. Some strains of group A b-hemolytic streptococci (Streptococcus pyogenes) and groups C and G streptococci may be inhibited in vitro by aztreonam concentrations of 6.25-32 mcg/mL, but the MIC90 (minimum inhibitory concentration at which 90% of strains tested are inhibited) of the drug for these organisms is usually 12.-64 mcg/mL. Group B streptococci (S. agalactiae), viridans streptococci, nonenterococcal group D streptococci, and enterococci are resistant to aztreonam. Penicillinase-producing, nonpenicillinase-producing, and methicillin-resistant strains of Staphylococcus aureus, S. epidermidis, and S. saprophyticus are resistant to aztreonam. Listeria monocytogenes and Nocardia also are resistant to the drug.
Gram-negative Aerobic Bacteria
Neisseria
Aztreonam is active in vitro against Neisseria meningitidis and N. gonorrhoeae. The MIC90 of aztreonam is 0.03-0.06 mcg/mL for N. meningitidis and 0.06-0.25 mcg/mL for both penicillinase- and nonpenicillinase-producing N. gonorrhoeae.
Haemophilus
Aztreonam is active in vitro against both b-lactamase- and non-b-lactamase-producing Haemophilus influenzae, and the MIC90 of the drug reported for these organisms is 0.06-0.25 mcg/mL. Aztreonam generally is active in vitro against strains of H. influenzae resistant to ampicillin and/or chloramphenicol as well as some strains resistant to ampicillin, chloramphenicol, and co-trimoxazole.
Moraxella catarrhalis
Both b-lactamase- and non-b-lactamase-producing strains of Moraxella catarrhalis (formerly Branhamella catarrhalis) generally are inhibited by aztreonam concentrations of 0.5-2 mcg/mL, and the MIC90 of the drug for this organism is 2 mcg/mL.
Enterobacteriaceae
Aztreonam is active in vitro against many Enterobacteriaceae. Citrobacter diversus, Enterobacter agglomerans, Escherichia coli, Hafnia alvei, Klebsiella pneumoniae, Morganella morganii, Proteus mirabilis, P. vulgaris, Providencia, Serratia marcescens, Salmonella, andShigella generally are inhibited in vitro by aztreonam concentrations of 4 mcg/mL or less. The in vitro activity of aztreonam against Citrobacter freundii, Enterobacter aerogenes, and E. cloacae varies considerably, and some strains of these organisms are resistant to the drug. Aztreonam is active in vitro against some, but not all, strains of Enterobacter, Klebsiella, and Serratia resistant to aminoglycosides
. Strains of Enterobacter resistant to both cefamandole and ticarcillin may also be resistant to aztreonam. The following table includes MIC50s (minimum inhibitory concentration at which 50% of strains tested are inhibited) and MIC90s of aztreonam reported for Enterobacteriaceae: Organism MIC50 (mcg/mL) MIC90 (mcg/mL) Citrobacter spp. 0.06-2 0.7-48 C. diversus 0.03-0. 0.06-16 C. freundii 0.03-32 0.20-32 Enterobacter spp. 0.06-2 0.12-64 E. aerogenes 0.06-16 0.20-33 E. agglomerans 0.03-0. 0.06-0. E. cloacae 0.06-16 3.10-32 Escherichia coli 0.03-0. 0.06-4 Hafnia alvei 0.12 0.12 Klebsiella spp. 0.06-0. 0.12-1 K. oxytoca 0.05-0. 0.25-12. K. pneumoniae 0.03-0. 0.06-0. Morganella morganii 0.01-0. 0.05-32 Proteus mirabilis 0.01-0. 0.01-0. P. vulgaris 0.01-0. 0.03-0. Providencia spp. 0.03-0. 0.25-0. P. rettgeri 0.01-0. 0.06-0. P. stuartii 0.01-0. 0.03-0. Serratia spp. 0.06-0. 0.9-8 S. marcescens 0.06-0. 0.39-6. Salmonella spp. 0.03-0. 0.10-0. S. enteritidis 0.05-0. 0.10-0. S. typhi 0.02-0. 0.05-0. Shigella spp. 0.03-0. 0.06-5. Sh. flexneri 0.05 0.10 Sh. sonnei 0.03-0. 0.06-0. Yersinia enterocolitica 0.2-0. 0.5-3.
Pseudomonas
Aztreonam is active in vitro against most strains of Pseudomonas aeruginosa, and the MIC50 and MIC90 of the drug for the organism are 2-16 and 4-32 mcg/mL, respectively. In vitro on a weight basis, aztreonam is more active than cefotaxime, ceftriaxone, or extended-spectrum penicillins, but less active than ceftazidime or imipenem against Ps. aeruginosa. Aztreonam is active in vitro against some, but not all, aminoglycoside-resistant Ps. aeruginosa. In studies using amikacin-resistant strains of Ps. aeruginosa, the MIC90 of aztreonam was 32 mcg/mL for isolates that were resistant to amikacin because of decreased uptake of the aminoglycoside and 32 mcg/mL or greater for isolates that were resistant to amikacin because of aminoglycoside-modifying enzymes. Aztreonam has some activity in vitro against Ps. acidovorans and Ps. stutzeri; the MIC90 of the drug for these organisms is 4-32 mcg/mL. Ps. diminuta, Ps. fluorescens, and Ps. putida are resistant to aztreonam.
Other Gram-negative Aerobic Bacteria
Pasteurella multocida generally are inhibited in vitro by aztreonam concentrations of 0.02-0. mcg/mL. Some strains of Moraxella are inhibited in vitro by aztreonam concentrations of 0.25-4 mcg/mL, but the MIC90 of the drug for the organism is greater than 64 mcg/mL. The MIC50 of aztreonam reported for Acinetobacter calcoaceticus subsp. anitratus and A. calcoaceticus subsp. lwoffi is 8-25 mcg/mL, but the MIC90 is usually 32 mcg/mL or greater and most strains are considered resistant to the drug. Aeromonas hydrophila and Plesiomonas shigelloides generally are inhibited in vitro by aztreonam, and the MIC90 of the drug for these organisms is 0.03-3. mcg/mL.
Burkholderia cepacia (formerly Ps. cepacia) and Stenotrophomonas maltophilia (formerly Ps. maltophilia) are resistant to aztreonam. Alcaligenes xylosoxidans (formerly Achromobacter xylosoxidans), Alcaligenes faecalis, and A. denitrificans are resistant to aztreonam. Bordetella bronchiseptica, Brucella melitensis, Flavobacterium meningosepticum, and Legionella pneumophila also are resistant to the drug.
Anaerobic Bacteria
Aztreonam generally is inactive against gram-positive and -negative anaerobic bacteria including Clostridium perfringens, C. difficile, Eubacterium, Peptococcus, Peptostreptococcus, Fusobacterium, Veillonella, Bacteroides fragilis, and Prevotella melaninogenica (Bacteroides melaninogenicus). The MIC90 of aztreonam usually is 64 mcg/mL or greater for anaerobic bacteria. Resistance Aztreonam has a high degree of stability against hydrolysis by bacterial b-lactamases, including both plasmid-mediated and chromosomally mediated enzymes. The drug generally is more stable against inactivation by b-lactamases than is cefotaxime, ceftizoxime, or cefoperazone.
Aztreonam generally is stable against hydrolysis by b-lactamases classified as Richmond-Sykes types I, III (TEM-1, TEM-2, SHV-1), and V (PSE and OXA types). The drug is stable against hydrolysis by most chromosomally mediated Richmond-Sykes type I enzymes produced by Citrobacter, Enterobacter, Morganella,Proteus, Providencia, Serratia, and Proteus.
The drug also is stable against hydrolysis by staphylococcal b-lactamases and most b-lactamases produced by Bacteroides. Aztreonam is hydrolyzed by a chromosomally mediated Richmond-Sykes type IV enzyme (K-1) produced by some strains of Klebsiella oxytoca, and strains that produce this enzyme generally are resistant to the drug. Aztreonam also is hydrolyzed to some extent by PSE 2, a Richmond-Sykes type V plasmid-mediated b-lactamase produced by Ps. aeruginosa; the drug generally is stable against hydrolysis by PSE 1, 3, and 4.
A chromosomally mediated b-lactamase produced by Bacteroides (B1) can also slowly hydrolyze aztreonam. In vitro studies indicate that aztreonam is a poor inducer of b-lactamase production and generally does not derepress inducible, chromosomally mediated enzymes in gram-negative bacteria that possess these enzymes (e.g., some strains of Pseudomonas, Citrobacter, Enterobacter, and Serratia). Although aztreonam is relatively stable against hydrolysis by inducible b-lactamases, it has been suggested that the enzymes may inactivate the drug by binding to it and preventing access to penicillin-binding proteins. Therefore, gram-negative bacteria that possess these inducible b-lactamases may be resistant to aztreonam following derepression of the enzymes.
Resistance to aztreonam has been produced in vitro in some strains of E. cloacae initially susceptible to the drug, and aztreonam-resistant strains of the organism have emerged during aztreonam therapy. E. cloacae resistant to aztreonam may also be resistant to third-generation cephalosporins and extended-spectrum penicillins, but may be susceptible to imipenem. Aztreonam resistance in some strains of E. cloacae appears to be related to alterations in outer-membrane porin proteins and/or other factors that affect permeability of the organism to the drug.
Pharmacokinetics
Aztreonam exhibits linear, dose-independent pharmacokinetics. The pharmacokinetics of the drug after IV administration are best described by an open, linear, 2-compartment model and pharmacokinetics after IM administration are best described by an open, one-compartment model with first-order absorption and elimination. The pharmacokinetics of aztreonam in pediatric patients 9 months of age or older are similar to those in adults.
Absorption
Aztreonam is poorly absorbed from the GI tract, and bioavailability of the drug is less than 1% following oral administration. In healthy adults who received a single 500-mg oral dose of aztreonam, peak serum concentrations of the drug were attained approximately 2 hours after the dose and averaged 0.1-0. mcg/mL; serum concentrations were undetectable 8 hours after the dose.
Aztreonam is rapidly and completely absorbed following IM administration, and peak serum concentrations of the drug generally are attained within 1 hour after an IM dose. Although peak serum concentrations of aztreonam attained with an IM dose are slightly lower than those attained with an equivalent IV dose, serum aztreonam concentrations 1 hour or longer after dosing are similar.
Following IM administration of a single 500-mg aztreonam dose in healthy adults, serum concentrations of the drug average 19-23.6, 21-26.7,17.2-21.4, 8.6-11.2, 3.8-5.9,1.5-3.3, and 0.1-1. mcg/mL at 0.5, 1, 2, 4, 6, 8, and 12 hours, respectively, after the dose. IM administration of a single 1-g IM dose of aztreonam in healthy adults results in serum aztreonam concentrations averaging 46.5,18.4,8.2,3.5, and 0.7 mcg/mL at 1, 4, 6, 8, and 12 hours, respectively, after the dose. In healthy adults, IV injection over 2-3 minutes of a single 0.5-, 1-, or 2-g dose of aztreonam results in peak serum concentrations of the drug averaging 56.3-67.2, 72.5-125, or 242 mcg/mL, respectively. Serum concentrations 1, 4, 6, 8, and 12 hours after injection average 38-48.6, 11-13.2,4.9-6, 2.6-2.7, and 0.5-0. mcg/mL, respectively, after the 1-g dose and 91, 26, 13, 6, and 1.2 mcg/mL, respectively, after the 2-g dose.
Following IV infusion over 30 minutes of a single 0.5-, 1-, or 2-g dose of aztreonam in healthy adults, peak serum concentrations of the drug immediately following completion of the infusion average 54-65.5,90-164, or 204-255 mcg/mL, respectively. Serum aztreonam concentrations 1, 2, 4, 8, and 12 hours after completion of the infusion average 48.8, 35.1, 16.2, 3, and 0.9 mcg/mL, respectively, after the 1-g dose and 111, 66.9,35.5, 6-8.5, and 1.9 mcg/mL, respectively, after the 2-g dose.
Multiple-dose studies in adults with normal renal and hepatic function receiving an IM or IV aztreonam dosage of 0.5-1 g every 8 hours for 7 days indicate that neither peak nor trough serum concentrations of the drug increase after repeated dosing and that the drug does not accumulate. In healthy adults receiving 1- or 2-g doses of aztreonam IM or IV every 8 hours, steady-state trough serum concentrations of the drug average 1-1.8 or 2.5-3.8 mcg/mL, respectively. In children following IV injection over 3 minutes of a single 30-mg/kg dose of aztreonam, serum concentrations of the drug 15 minutes and 6 hours after the dose average 118. and 11. mcg/mL, respectively, in those 2-21 months of age and 96. and 5.8 mcg/mL, respectively, in those 2-12 years of age.
IV infusion over 10 minutes of 30-mg/kg doses of aztreonam every 12 hours in neonates 1-4 days of age weighing 0.5-2 kg results in peak serum aztreonam concentrations averaging 65.-83. mcg/mL. In a study in premature neonates who received a single 30-mg/kg dose of aztreonam given IV over 3 minutes, plasma concentrations of the drug 1 hour after the dose averaged 46. mcg/mL in those with a gestational age of less than 30 weeks who weighed less than 1.5 kg and 49. mcg/mL in those with a gestational age of more than 30 weeks who weighed more than 1.5 kg; plasma concentrations 8 hours after the dose averaged 14.1 and 14.83 mcg/mL, respectively.
In patients with chronic renal failure undergoing continuous ambulatory peritoneal dialysis, intraperitoneal administration of a single 1-g dose of aztreonam in 2 L of dialysis fluid instilled over 10 minutes results in serum aztreonam concentrations averaging 10.9,30,4 and 0.4 mcg/mL at 0.5, 6, 24, and 48 hours, respectively, after the dose.
Distribution
Aztreonam is widely distributed into body tissues and fluids following IM or IV administration.
The drug is distributed into skeletal muscle, adipose tissue, skin, bone, gallbladder, liver, lungs, kidneys, atrial appendage, intestines, prostatic tissue, myometrium, endometrium, fallopian tubes, ovaries, and cervical and vaginal tissue.
Aztreonam also is distributed into saliva, sputum, bronchial secretions, aqueous humor, and bile, and into pericardial, pleural, peritoneal, synovial, and blister fluids. In healthy adults, the apparent volume of distribution of aztreonam in the central compartment (Vc) averages 0.05-0. L/kg and the volume of distribution at steady state (Vss) averages 0.11-0.22 L/kg. The Vc and Vss of aztreonam in adults with mild to moderate renal impairment are similar to those in healthy adults; however, in one study in adults with severe renal impairment (creatinine clearances less than 10 mL/minute), the Vc and Vss of the drug were 0.13-0.38 and 0.24-0.67 L/kg, respectively.
The Vss of aztreonam averages 0.26-0. L/kg in neonates and 0.2-0.29 L/kg in children 1 month to 12 years of age. The Vss of the drug may be increased in patients with cystic fibrosis. Aztreonam is distributed into CSF following IV administration. CSF concentrations of aztreonam generally are higher in patients with inflamed meninges than in those with uninflamed meninges; the ratio of CSF/serum concentration is generally 0.03-0.52 in patients with inflamed meninges and 0.02-0.30 in patients with uninflamed meninges. In adults who received a single 2-g dose of aztreonam given by IV injection over 5 minutes, CSF concentrations of the drug 1 and 4 hours after the dose were 2 and 3.2 mcg/mL, respectively, in those with inflamed meninges and 0.5 and 1 mcg/mL, respectively, in those with uninflamed meninges. In a neonate and several children 3 months to 2 years of age with bacterial meningitis who received a single 30-mg/kg dose of aztreonam by IV injection over 3 minutes, CSF aztreonam concentrations ranged from 2.1-20.8 mcg/mL in samples obtained 0.8-4. hours after the dose and the CSF/serum ratio ranged from 0.06-0.24.
Aztreonam concentrations in peritoneal fluid are approximately equal to concurrent serum concentrations of the drug. In patients receiving 2-g doses of aztreonam, peritoneal fluid concentrations 1-6 hours after a dose ranged from 12-90 mcg/mL. In several patients with abdominal infections, peritoneal fluid concentrations were about 50% lower in those with purulent fluid than in those with clear, serous fluid.
In adults who received a single 2-g dose of aztreonam by IV injection over 5 minutes, aztreonam concentrations approximately 1 hour after the dose averaged 362. mcg/mL in common duct bile, 102.8 mcg/mL in gallbladder bile, 27. mcg/g in gallbladder, and 89. mcg/mL in serum. In patients undergoing cholecystectomy who received a single 1-g IV dose of aztreonam, peak concentrations of the drug in T-tube biliary drainage were attained approximately 2.4 hours after the dose and ranged from 9.7-88. mcg/mL; biliary concentrations ranged from 5.4-39.8 mcg/mL at 6 hours after the dose.
When a single 1-g IV dose of aztreonam was given to patients with biliary obstruction, peak concentrations of the drug in bile were attained 1 hour after the dose and ranged from 5.6-23. mcg/mL. At serum concentrations of 1-100 mcg/mL, aztreonam is 46-60% bound to serum proteins in healthy adults. In adults with impaired renal function and decreased serum albumin concentrations, aztreonam is 22-49% bound to serum proteins.
Aztreonam crosses the placenta and is distributed into amniotic fluid. Aztreonam concentrations in amniotic fluid have been reported to be 2 mcg/mL 6-8 hours after a single 1-g IV dose. The drug is distributed into milk in low concentrations. In lactating women who received a single 1-g IM or IV dose of aztreonam, peak milk concentrations were attained 2-6 hours after the dose and averaged 0.2 or 0.3 mcg/mL, respectively, and concurrent serum concentrations averaged 126 or 43 mcg/mL, respectively.
Elimination
Aztreonam is partially metabolized to several microbiologically inactive metabolites; no active metabolites of the drug have been found in serum or urine. The principal metabolite of aztreonam, which is formed by nonspecific hydrolysis of the b-lactam ring, is 2-[[(2-amino-4-thiazolyl)[(1-carboxy-1-methylethoxy) imino]acetyl]amino]-3-(sulfoamino)butanoic acid (SQ 26,).
Other inactive metabolites, which have not been identified, reportedly may be demethylated products of SQ 26,992. Serum concentrations of aztreonam decline in a biphasic manner after IV administration. In adults with normal renal and hepatic function, the distribution half-life (t1/2a) of aztreonam averages 0.2-0.7 hours and the elimination half-life (t1/2b) averages 1.3-2. hours. The t1/2b of SQ 26, is longer than that of aztreonam and is about 26 hours in adults with normal renal and hepatic function.
The t1/2b of aztreonam is slightly longer in geriatric adults than in younger adults and ranges from 1.7-4.3 hours in adults 64-82 years of age with renal function normal for their age. The t1/2b of aztreonam averages 1.7 hours in children 2 months to 12 years of age.
Half-life of the drug is longer in neonates than in older children and adults and is inversely related to age and birthweight. In neonates younger than 7 days of age, t1/2b of aztreonam averages 5.5-9.9 hours in those weighing less than 2.5 kg and 2.6 hours in those weighing more than 2.5 kg. In neonates 1 week to 1 month of age, t1/2? of the drug averages 2.4 hours. Serum concentrations of aztreonam are higher and the serum half-life prolonged in patients with renal impairment. In adults with renal impairment, the t1/2b of aztreonam averages 3.4-3.6,5.3-5.9, 7.8-7.9, or 8.4-8.7. hours in adults with creatinine clearances of 30-80, 10-29, 3-9, or less than 2 mL/minute, respectively.
Half-life of aztreonam is only slightly prolonged in patients with hepatic impairment, since the liver is a minor pathway of elimination for the drug. In a study in patients with cirrhosis but with normal renal function, the t1/2b of aztreonam averaged 2.2 hours in those with primary biliary cirrhosis and 3.2 hours in those with alcoholic cirrhosis. Aztreonam is excreted principally in urine as unchanged drug via both glomerular filtration and tubular secretion.
Following IM or IV administration of a single 0.5-, 1-, or 2-g dose of aztreonam in adults with normal renal function, approximately 58-74% of the dose is excreted in urine unchanged, 1-7% is excreted as SQ 26,992, and 3-4% is excreted as unidentified inactive metabolites. Urinary excretion of unchanged aztreonam is essentially complete 8-12 hours after a single dose of the drug, but SQ 26, is excreted for up to 48 hours after the dose.
Following IM administration of a single 0.5- or 1-g dose of aztreonam in adults with normal renal function, urinary concentrations of the drug average 500 or 1200 mcg/mL, respectively, in urine collected over the first 2 hours after the dose and 180 or 470 mcg/mL, respectively, in urine collected over 6-8 hours after the dose. In adults with normal renal function, urinary concentrations of aztreonam after a single 0.5- or 1-g dose given by IV injection over 3-5 minutes or IV infusion over 30 minutes average 1-1. or 3-3. mg/mL, respectively, in urine collected over the first 2 hours after the dose and 250-330 or 710-720 mcg/mL, respectively, in urine collected over 4-6 hours after the dose. After a single 2-g IV dose, urinary concentrations of aztreonam average 5.6-6. mg/mL in urine collected over the first 2 hours after the dose and 1.8 mg/mL in urine collected over 4-6 hours after the dose. Aztreonam is partially excreted in feces, presumably via biliary elimination.
Approximately 1% of a single 500-mg IV dose of the drug is excreted in feces unchanged, 3% as SQ 26,, and 7.5-10.% as unidentified inactive metabolites. Total body clearance of aztreonam from serum averages 0.91-1.68 mL/minute per kg and renal clearance of the drug averages 0.59-1.03 mL/minute per kg in healthy adults. Total body clearance from serum averages 0.61-1.13 mL/minute per kg in neonates younger than 7 days of age weighing less than 2.5 kg, 1.41-1.68 mL/minute per kg in neonates weighing more than 2.5 kg, 1.87 mL/minute per kg in children 2-21 months of age, and 2.5 mL/minute per kg in children 2-12 years of age. Total body clearance from serum in adults with impaired renal function decreases linearly with decreases in creatinine clearance. Cystic fibrosis patients may eliminate aztreonam at a faster rate than healthy individuals. Serum half-life of the drug averaged 1-1.3 hours in several patients with cystic fibrosis.
Further study is needed to determine if this effect is clinically important since use of usual dosages of the drug in patients with cystic fibrosis may result in lower serum concentrations than expected. Aztreonam and SQ 26, are removed by hemodialysis. The amount of the drug and its metabolites removed during hemodialysis depends on several factors (e.g., type of coil used, dialysis flow rate). In one group of patients with end-stage renal disease undergoing hemodialysis, the serum half-life of aztreonam averaged 2.7 hours during hemodialysis and 7.9 hours between dialysis sessions.
A 4-hour period of hemodialysis generally removes into the dialysate about 27-58% of a single 1-g IV dose of aztreonam when the dose is given 1 hour prior to dialysis. Aztreonam is removed to a lesser extent by peritoneal dialysis. In patients with chronic renal failure undergoing continuous ambulatory peritoneal dialysis with a 6-hour dwell time, about 10% of a single 1-g IV dose of aztreonam is removed into the dialysate within 48 hours after the dose.
Chemistry and Stability
Chemistry
Aztreonam is a synthetic monobactam antibiotic.
Unlike other currently available b-lactam antibiotics, which are bicyclic and contain an adjoining ring fused to the b-lactam ring (e.g., carbapenems, cephalosporins, cephamycins, 1-oxa-b-lactams, penicillins), monobactams are monocyclic b-lactam antibiotics. Naturally occurring monobactams are produced by various bacteria found in soil (e.g., Acetobacter, Agrobacterium, Chromobacterium, Flexibacter, Gluconobacter) and generally have only weak antibacterial activity. Synthetic monobactams, including aztreonam, contain a 3-aminomonobactamic acid (3-AMA) nucleus; addition of various substituents on the 3-AMA nucleus results in monobactam derivatives that differ in spectra of activity, antimicrobial potency, and stability against hydrolysis by b-lactamases.
Aztreonam contains a sulfonic acid group on the nitrogen at the 1-position of the 3-AMA nucleus, which activates the b-lactam ring. The drug contains an aminothiazolyl oxime side chain at position 3 of the 3-AMA nucleus, which results in potent activity against gram-negative bacteria; the carboxyl and methyl groups on the side chain result in enhanced activity against Pseudomonas aeruginosa and decreased activity against gram-positive bacteria. This aminothiazolyl side chain is similar to that contained in ceftazidime. In addition, aztreonam contains an a-methyl group at position 4 of the 3-AMA nucleus, which results in stability against hydrolysis by many b-lactamases and increased antibacterial potency against gram-negative bacteria, but decreased activity against gram-positive bacteria.
Commercially available aztreonam for injection is a dry mixture of sterile aztreonam and arginine, and occurs as a white to yellowish-white, lyophilized cake; the injection contains approximately 780 mg of arginine per gram of aztreonam. Aztreonam has solubilities of 10 mg/mL in water and 0.2 mg/mL in alcohol at 25°C. The drug has pKas of -0.7, 2.75, and 3.91 at 25°C. Aqueous solutions of aztreonam have a pH of 4.5-7..
Potency of aztreonam powder for injection is calculated on the anhydrous and arginine-free basis. When reconstituted as directed, solutions of aztreonam are colorless to light straw yellow depending on the concentration of the drug and the diluent used. Aztreonam solutions may turn slightly pink on standing; this color change does not indicate loss of potency. The commercially available frozen aztreonam in dextrose injections are light yellow- to amber-colored, nonpyrogenic, iso-osmotic, sterile solutions of the drug; about 1.7 or 0.7 g of dextrose has been added to the 1- or 2-g injections of aztreonam, respectively, to adjust osmolality to about 300 mOsm/kg and about 0.78 or 1.6 g of arginine has been added to the 1- or 2-g injections of aztreonam, respectively, to adjust pH to 4.5-7.5.
Stability
Aztreonam powder for injection should be stored at room temperature and should not be exposed to temperatures warmer than 40°C. The powder for injection also should be protected from moisture. If exposed to strong light, the powder may yellow slightly. Aztreonam is most stable at pH 6.244, 245 In acidic solutions with pH 2-5, aztreonam is inactivated by isomerization of the oxime side chain and hydrolysis of the b-lactam ring.
The drug also is inactivated in solutions with pH less than 1 or greater than 8.245 Following reconstitution with sterile water for injection or 0.9% sodium chloride injection, IM injections of aztreonam are stable for 48 hours at 15-30°C or for 7 days when refrigerated at 2-8°C. IM injections of the drug reconstituted with bacteriostatic water for injection (with benzyl alcohol or parabens) or bacteriostatic sodium chloride injection (with benzyl alcohol or parabens) should be used immediately after reconstitution. Aztreonam solutions can be frozen immediately after preparation and are stable for up to 3 months at -20°C.
Frozen solutions may be thawed either at 15-30°C or by overnight refrigeration. Frozen solutions that have been thawed and stored at room temperature should be used within 24 hours, and those that have been thawed and refrigerated at 2-8°C should be used within 72 hours after removal from the freezer. Once thawed, aztreonam solutions should not be refrozen. The manufacturer states that the stability of the commercially available frozen aztreonam injection may vary.
These injections are stable for at least 90 days from the date of shipment when stored at -20°C. The frozen injection should be thawed at room temperature (25°C) or under refrigeration (2-8°C) and, once thawed, should not be refrozen. Thawed solutions of the commercially available frozen injection are stable for 48 hours at room temperature (25°C) or 14 days when refrigerated at 5°C.
Aztreonam frozen injections should be removed from their original carton according to the manufacturer’s directions and may be thawed individually; when thawing is complete, the container should be inverted to assure a well-mixed solution. The manufacturer states that aztreonam frozen injections are single-dose units and unused contents of any container should be discarded. The commercially available frozen injection of the drug is provided in a plastic container fabricated from specially formulated polyvinyl chloride (PL 146® plastic).
Solutions in contact with the plastic can leach out some of its chemical components in very small amounts (e.g., bis(2-ethylhexyl)phthalate [BEHP, DEHP] in up to 5 ppm) within the expiration period of the injection; however, safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies. At a concentration of 20 mg or less per mL, aztreonam is chemically and physically stable for 48 hours at 15-30°C or for 7 days when refrigerated at 2-8°C in the following IV infusion solutions: 0.9% sodium chloride injection; 5 or 10% dextrose injection; Ringer’s; lactated Ringer’s; 5% dextrose and 0.2, 0.45, or 0.9% sodium chloride; 1/6 M sodium lactate; 5 or 10% mannitol; 5% dextrose with lactated Ringer’s; 5% dextrose with Plasma-Lyte® M; 10% Travert®; 10% Travert® with Electrolyte No. 1, 2, or 3; Ionosol® B with 5% dextrose; Isolyte® E; Isolyte® E with 5% dextrose; Isolyte® M with 5% dextrose; Normosol®-R; Normosol®-R with 5% dextrose; or Normosol®-M with 5% dextrose.
At concentrations greater than 20 mg/mL, aztreonam is stable for 48 hours at 2-8°C in sterile water for injection or 0.9% sodium chloride injection; solutions containing aztreonam concentrations greater than 20 mg/mL prepared using other compatible IV solutions should be used immediately. Admixtures containing aztreonam (10 or 20 mg/mL) and clindamycin phosphate (3, 6, or 9 mg/mL) or cefazolin (5 or 20 mg/mL) in 0.9% sodium chloride injection or 5% dextrose injection are stable for up to 48 hours at room temperature (25°C) or for 7 days when refrigerated at 4-5°C.
Admixtures containing aztreonam (10 or 20 mg/mL) and ampicillin (5 or 20 mg/mL) in 0.9% sodium chloride injection are stable for 24 hours at room temperature (25°C) or for 48 hours when refrigerated at 4°C; similar admixtures of these drugs in 5% dextrose injection are stable for only 2 hours at room temperature or for 8 hours when refrigerated at 4°C. Admixtures containing aztreonam (10 or 20 mg/mL) and cefoxitin (10 or 20 mg/mL) in one of these diluents are stable for 12 hours at 25°C or 7 days at 4°C. Although many bicyclic b-lactam antibiotics (e.g., penicillins) are physically and/or chemically incompatible with aminoglycosides and can inactivate the drugs in vitro, aztreonam appears to be less likely to inactivate aminoglycosides in vitro. Admixtures containing aztreonam (10 or 20 mg/mL) and tobramycin (0. or 0.8 mg/mL) in 0.9% sodium chloride injection or 5% dextrose injection are stable for up to 48 hours at room temperature (25°C) or for 7 days at 4°C.
The manufacturer states that similar solutions containing gentamicin (0. or 0.8 mg/mL) are also stable for up to 48 hours at room temperature or for 7 days when refrigerated. However, there is evidence that loss of gentamicin potency may occur with such admixtures at these storage temperatures and times; therefore, it has been suggested that solutions containing aztreonam (10 or 20 mg/mL) and gentamicin (0.2 or 0.8 mg/mL) be considered stable for only 8 hours at 25°C or for 24 hours at 4°C.
Admixtures containing aztreonam and either cloxacillin sodium or vancomycin hydrochloride in Dianeal® 137 with 4.25% dextrose are stable for up to 24 hours at room temperature. Aztreonam is physically and/or chemically incompatible with some drugs, but the compatibility depends on several factors (e.g., concentrations of the drugs, specific diluents used, resulting pH, temperature).
Specialized references should be consulted for specific compatibility information. Aztreonam is incompatible with nafcillin sodium, cephradine, or metronidazole, and these drugs should be administered separately.
Preparations
Aztreonam Parenteral For injection 500 mg Azactam®, (with arginine) Elan 1 g Azactam®, (with arginine) Elan 2 g Azactam®, (with arginine) Elan For injection, for 1 g Azactam®, (with arginine) IV infusion Elan 2 g Azactam®, (with arginine) Elan Aztreonam in Dextrose Parenteral Injection (frozen) 20 mg/mL (1 g) in 3.4% Azactam® in Iso-osmotic , for IV infusion Dextrose Dextrose Injection, (Galaxy® [Baxter]) Elan 40 mg/mL (2 g) in 1.4% Azactam® in Iso-osmotic Dextrose Dextrose Injection Galaxy®, (Galaxy® [Baxter]) Elan

