Ceftizoxime is a semisynthetic, third generation cephalosporin antibiotic.
Uses
Ceftizoxime is used for the treatment of bone and joint infections, intra-abdominal infections, meningitis, lower respiratory tract infections, septicemia, skin and skin structure infections, and urinary tract infections caused by susceptible bacteria. The drug also is used for the treatment of gonorrhea and pelvic inflammatory disease.In addition, ceftizoxime has been used for perioperative prophylaxis.
Prior to initiation of ceftizoxime therapy, appropriate specimens should be obtained for identification of the causative organism and in vitro susceptibility tests. Ceftizoxime therapy may be started pending results of susceptibility tests but should be discontinued if the causative organism is found to be resistant to the drug. In serious infections when the causative organism is unknown, concomitant therapy with an aminoglycoside may be indicated pending results of in vitro susceptibility tests.
Gram-positive Aerobic Bacterial Infections
Ceftizoxime is used in the treatment of lower respiratory tract infections caused by susceptible Streptococcus pneumoniae, other streptococci (except enterococci), or Staphylococcus aureus; urinary tract infections caused by susceptible S. aureus; intra-abdominal infections caused by susceptible S. epidermidis or streptococci (except enterococci); skin and skin structure infections caused by susceptible S. aureus, S. epidermidis, S. pyogenes (group A b-hemolytic streptococci), or other streptococci (except enterococci); bone and joint infections caused by susceptible S. aureus or streptococci (except enterococci); and septicemia caused by susceptible S. pneumoniae, other streptococci (except enterococci), or S. aureus.Ceftizoxime generally should not be used in the treatment of infections caused by gram-positive bacteria when a penicillin or a first generation cephalosporin could be used.
Gram-negative Aerobic Bacterial Infections
Ceftizoxime has been effective when used in the treatment of lower respiratory tract infections caused by susceptible Escherichia coli, Klebsiella, Haemophilus influenzae, Proteus mirabilis, Serratia, or Enterobacter; urinary tract infections caused by susceptible Enterobacter, E. coli, Klebsiella, P. mirabilis, Morganella morganii, Providencia rettgeri, P. vulgaris, Pseudomonas (including Ps. aeruginosa), or Serratia (including S. marcescens); intra-abdominal infections caused by susceptible Enterobacter, E. coli, or Klebsiella; skin and skin structure infections caused by susceptible Enterobacter, E. coli, Klebsiella, P. mirabilis, or Serratia; bone and joint infections caused by susceptible P. mirabilis; septicemia caused by susceptible E. coli, Klebsiella, or Serratia; or uncomplicated cervical and urethral gonorrhea caused by susceptible Neisseria gonorrhoeae.
It has been suggested that certain parenteral cephalosporins (i.e., cefepime, cefotaxime, ceftizoxime, ceftriaxone, ceftazidime) may be drugs of choice for the treatment of many infections caused by susceptible strains of E. coli, K. pneumoniae, P. rettgeri, M. morganii, P. vulgaris, P. stuartii, or Serratia; an aminoglycoside should be used concomitantly in severe infections. The choice of anti-infective agent for the treatment of sepsis syndrome should be based on the probable source of infection, gram-stained smears of appropriate clinical specimens, the immune status of the patient, and current patterns of bacterial resistance within the hospital and local community.
Certain parenteral cephalosporins (i.e., cefepime, cefotaxime, ceftizoxime, ceftriaxone, ceftazidime) are good choices for the treatment of gram-negative sepsis. For the initial treatment of life-threatening sepsis in adults, some clinicians suggest use of a parenteral cephalosporin (i.e., cefepime, cefotaxime, ceftriaxone) given in conjunction with an aminoglycoside. The manufacturer states that, because high concentrations of ceftizoxime are attained in urine, the drug may be used alone in the treatment of urinary tract infections caused by susceptible Ps. aeruginosa provided that high dosage is used and other anti-infective therapy is substituted if clinical response to the drug is not prompt. However, because many strains of Ps. aeruginosa are only susceptible to high concentrations of ceftizoxime in vitro, many clinicians state that ceftizoxime should not be used alone in the treatment of any infection where Ps. aeruginosa may be present.
Anaerobic and Mixed Aerobic-Anaerobic Bacterial Infections
Ceftizoxime is used in the treatment of lower respiratory tract, intra-abdominal, or skin and skin structure infections caused by susceptible Bacteroides (including B. fragilis) and in the treatment of skin and skin structure infections caused by susceptible anaerobic cocci (including Peptococcus and Peptostreptococcus). Ceftizoxime has been effective when used in the treatment of mixed aerobic-anaerobic infections including intra-abdominal and gynecologic infections (see Uses: Pelvic Inflammatory Disease).
Gonorrhea and Associated Infections
Uncomplicated Gonorrhea
Ceftizoxime is used for the treatment of uncomplicated cervical, urethral, or rectal gonorrhea caused by penicillinase-producing strains of Neisseria gonorrhoeae (PPNG) or nonpenicillinase-producing strains of the organism. However, ceftizoxime is considered an alternative agent for the treatment of uncomplicated gonorrhea. The US Centers for Disease Control and Prevention (CDC) and most clinicians currently recommend that uncomplicated cervical, urethral, or rectal gonorrhea in adults and adolescents be treated with a single IM dose of ceftriaxone, a single oral dose of cefixime, or a single oral dose of certain fluoroquinolones (ciprofloxacin, ofloxacin, or levofloxacin) given in conjunction with an anti-infective regimen effective for presumptive treatment of chlamydia (e.g., a single dose of oral azithromycin or a 7-day regimen of oral doxycycline).
However, fluoroquinolones should not be used for the treatment of gonorrhea acquired in Asia or the Pacific islands (including Hawaii) and may be inadvisable for infections acquired in other areas when N. gonorrhoeae with quinolone resistance have been reported (including California). (See Uses: Gonorrhea and Associated Infections, in Ciprofloxacin 8:12.18.) Alternative regimens that are recommended by the CDC for the treatment of uncomplicated cervical, urethral, or rectal gonorrhea in adults and adolescents include a single IM dose of spectinomycin, a single IM dose of certain cephalosporins (cefotaxime, cefoxitin, ceftizoxime), or a single oral dose of certain fluoroquinolones (gatifloxacin, lomefloxacin, norfloxacin) given in conjunction with an anti-infective regimen effective for presumptive treatment of chlamydia. Although a single 500-mg IM dose of ceftizoxime may be effective in the treatment of uncomplicated urogenital and anorectal gonorrhea, the CDC states that the drug does not appear to offer any advantage over ceftriaxone for the treatment of gonorrhea.
Disseminated Gonococcal Infections
IV ceftizoxime is recommended by the CDC as one of several acceptable alternative regimens for initial treatment of disseminated gonococcal infections in adults and adolescents. The CDC currently recommends that treatment of disseminated gonococcal infections in adults and adolescents be initiated with a multiple-dose regimen of IM or IV ceftriaxone. Alternative initial regimens recommended by the CDC for disseminated gonococcal infections include multiple-dose parenteral regimens of certain IV cephalosporins (cefotaxime, ceftizoxime), certain IV fluoroquinolones (ciprofloxacin, levofloxacin), or IM spectinomycin.
The initial parenteral regimen should be continued for 24-48 hours after improvement begins; therapy can then be switched to oral cefixime, oral ciprofloxacin, oral ofloxacin, or oral levofloxacin and continued to complete at least 1 week of treatment. The CDC recommends that the patient be hospitalized for initial treatment, especially when compliance may be a problem, when the diagnosis is uncertain, or when the patient has purulent synovial effusions or other complications. Patients should be examined for clinical evidence of endocarditis and meningitis; the recommended regimens for these infections is IV ceftriaxone. An anti-infective regimen effective for presumptive treatment of chlamydia should be given in conjunction with treatment for disseminated gonococcal infections unless the presence of coexisting chlamydial infection has been excluded by appropriate testing.
For additional information on current recommendations regarding the treatment of gonorrhea and associated infections, see Uses: Gonorrhea and Associated Infections in Ceftriaxone 8:12.06.12.
Pelvic Inflammatory
Disease Ceftizoxime is used for the treatment of pelvic inflammatory disease (PID). Although experience is limited, results of several randomized comparative clinical studies indicate that IV regimens of ceftizoxime/doxycycline, cefoxitin/doxycycline, or clindamycin/gentamicin appear to be comparably effective for the treatment of acute PID involving Neisseria gonorrhoeae as a principal causative agent.
Because ceftizoxime (like other cephalosporins) has no activity against Chlamydia trachomatis, it should be given in conjunction with an anti-infective active against this organism (e.g., doxycycline) whenever it is used in the treatment of PID. When a parenteral regimen is indicated for the treatment of PID, the CDC and other clinicians generally recommend a regimen of cefotetan (2 g IV every 12 hours) or cefoxitin (2 g IV every 6 hours) given in conjunction with doxycycline (100 mg IV or orally every 12 hours) or a regimen of clindamycin (900 mg IV every 8 hours) given in conjunction with gentamicin (usually a 2-mg/kg IV or IM loading dose followed by 1.5 mg/kg every 8 hours).
While there is some evidence that other parenteral cephalosporins (e.g., ceftizoxime, cefotaxime, ceftriaxone) also may be effective for the treatment of PID, the CDC states that there is less experience with use of these cephalosporins in patients with PID and these drugs may be less active than cefotetan or cefoxitin against anaerobic bacteria.
Traditionally, parenteral regimens for the treatment of PID have been continued for at least 48 hours after the patient demonstrates substantial clinical improvement and then an oral regimen is continued to complete a total of 14 days of therapy; however, the CDC states that a transition to oral therapy may occur within 24 hours after the patient demonstrates clinical improvement and that decisions regarding such a transition should be guided by clinical experience. Most clinicians recommend at least 24 hours of direct inpatient observation for patients with tubo-ovarian abscesses after which time anti-infective therapy at home is adequate. For additional information on treatment of PID, including information on follow-up and management of sexual partners, see Uses: Pelvic Inflammatory Disease, in the Cephalosporins General Statement 8:12.06.)
Meningitis
Ceftizoxime has been effective when used alone in adults or pediatric patients for the treatment of meningitis caused by susceptible Haemophilus influenzae, Neisseria meningitidis, S. pneumoniae, or E. coli. However, cefotaxime or ceftriaxone generally is preferred when a cephalosporin is indicated for the treatment of bacterial meningitis caused by these organisms. For information on use of cephalosporins in the treatment of bacterial meningitis, see Uses: Meningitis and Other CNS Infections in the Cephalosporins General Statement 8:12.06.
Perioperative Prophylaxis
IV ceftizoxime has been effective when used for perioperative prophylaxis in patients undergoing biliary tract or colorectal surgery. Although results of a randomized, double-blind study indicate that ceftizoxime may be as effective as cefazolin for the prevention of postoperative infections in adults undergoing elective biliary tract surgery, cefazolin generally is considered the drug of choice for high risk patients undergoing biliary tract surgery. In addition, while results of one study suggested that ceftizoxime may be more effective than cefoxitin for perioperative prophylaxis in patients undergoing elective colorectal surgery, cefoxitin or cefotetan or, alternatively, a regimen of cefazolin and metronidazole generally is recommended when a parenteral regimen is used for perioperative prophylaxis in patients undergoing colorectal surgery.
Some clinicians state that third generation cephalosporins (e.g., cefoperazone, cefotaxime, ceftriaxone, ceftazidime, ceftizoxime) and fourth generation cephalosporins (e.g., cefepime) should not be used for perioperative prophylaxis since they are expensive, some are less active against staphylococci than cefazolin, they have a spectrum of activity that is wider than necessary for organisms encountered in elective surgery, and their use for prophylaxis promotes emergence of resistant organisms. (See Uses: Perioperative Prophylaxis, in the Cephalosporins General Statement 8:12.06.)
Dosage and Administration
Reconstitution and Administration
Ceftizoxime sodium is administered by direct IV or deep IM injection or by IV infusion. The drug should be given IV rather than IM in patients with septicemia, localized parenchymal abscesses (e.g., intra-abdominal abscess), peritonitis, or other severe or life-threatening infections.
Intermittent IV Injection
For direct intermittent IV injection, 5, 10, or 20 mL of sterile water for injection should be added to a vial labeled as containing 500 mg, 1 g, or 2 g of ceftizoxime, respectively, to provide solutions containing approximately 95 mg/mL. The appropriate dose should then be injected directly into a vein over a 3- to 5-minute period or injected slowly into the tubing of a freely flowing compatible IV solution. (See Chemistry and Stability: Stability.)
Intermittent or Continuous IV Infusion
For intermittent or continuous IV infusion, 50-100 mL of 0.9% sodium chloride injection, 5% dextrose injection, or other compatible IV solution may be added to a piggyback vial labeled as containing 1 or 2 g of ceftizoxime.
Alternatively, reconstituted solutions of ceftizoxime may be added to an IV container containing 50-100 mL of a compatible IV solution. Thawed solutions of the commercially available frozen ceftizoxime sodium injection should be administered only by intermittent or continuous IV infusion. After thawing at room temperature, the container should be checked for minute leaks by firmly squeezing the bag. The injection should be discarded if the container seal is not intact or leaks are found or if the solution is cloudy or contains a precipitate. Additives should not be introduced into the injection container.
The injection should not be used in series connections with other plastic containers, since such use could result in air embolism from residual air being drawn from the primary container before administration of fluid from the secondary container is complete. Intermittent IV infusions of ceftizoxime are generally infused over 15-30 minutes.
IM Injection
IM injections of ceftizoxime are prepared by adding 1.5, 3, or 6 mL of sterile water for injection to a vial labeled as containing 500 mg, 1 g, or 2 g of ceftizoxime, respectively, to provide solutions containing approximately 280, 270, or 270 mg/mL, respectively. IM injections should be made deeply into a large muscle; when administering 2-g doses of ceftizoxime IM, the dose should be divided and given in 2 different large muscle masses. The plunger of the syringe should be drawn back before IM injection to ensure that the needle is not in a blood vessel.
Dosage
Dosage of ceftizoxime sodium is expressed in terms of ceftizoxime and is identical for IM or IV administration. Adult Dosage The usual adult dosage of ceftizoxime is 1 or 2 g every 8-12 hours. Uncomplicated urinary tract infections in adults generally respond to 500 mg every 12 hours.
The manufacturer states that higher dosage should be used if ceftizoxime is used in the treatment of urinary tract infections caused by susceptible Ps. aeruginosa. (See Uses: Gram-negative Aerobic Bacterial Infections.) Infections in adults caused by other susceptible bacteria generally respond to 1 g every 8-12 hours; however, severe or complicated infections may require 1 g every 8 hours or 2 g every 8-12 hours. Life-threatening infections in adults may require 3-4 g IV every 8 hours; dosages up to 2 g every 4 hours have been given.
For the treatment of septicemia caused by susceptible bacteria, the manufacturer states that ceftizoxime therapy should be initiated with an IV dosage of 6-12 g daily, and dosage gradually decreased according to the clinical response of the patient and bacteriologic assessments.
Gonorrhea and Associated Infections
For the treatment of uncomplicated gonorrhea caused by penicillinase-producing strains of N. gonorrhoeae (PPNG) or nonpenicillinase-producing strains of the organism, the CDC recommends that adults receive a single 500-mg IM dose of ceftizoxime; the manufacturer recommends a single 1-g IM dose of the drug. For the treatment of disseminated gonococcal infections, the CDC recommends that adults receive 1 g of ceftizoxime IV every 8 hours continued for 24-48 hours after improvement begins; therapy may then be switched to an oral regimen of cefixime, ciprofloxacin, ofloxacin, or levofloxacin to complete at least 1 week of therapy. Unless the presence of coexisting chlamydial infection has been excluded by appropriate testing, ceftizoxime therapy for uncomplicated or disseminated gonococcal infections should be administered in conjunction with an anti-infective regimen effective for presumptive treatment of chlamydia (e.g., a single dose of oral azithromycin or a 7-day regimen of oral doxycycline).
Pelvic Inflammatory Disease
For the treatment of pelvic inflammatory disease (PID) caused by Neisseria gonorrhoeae, Escherichia coli, or Streptococcus agalactiae (group B streptococci), hospitalized adults can receive an IV ceftizoxime dosage of 2 g every 8 hours (6 g daily). For possible coexisting chlamydial infection, IV ceftizoxime is given in conjunction with oral or IV doxycycline 100 mg every 12 hours for at least 24 hours followed by oral doxycycline 100 mg twice daily to complete 14 days of therapy.
Pediatric Dosage
The usual dosage of ceftizoxime for children 6 months of age and older is 50 mg/kg every 6-8 hours; a total daily dose of 200 mg/kg given in divided doses may be necessary for severe infections, but dosage should not exceed 12 g daily. Although safe use of ceftizoxime in children younger than 6 months has not been definitely established, the American Academy of Pediatrics (AAP) states that children older than 1 month of age may receive ceftizoxime in a dosage of 100-150 mg/kg daily given in 3 divided doses for the treatment of mild to moderate infections or a dosage of 150-200 mg/kg daily given in 3 or 4 divided doses for the treatment of severe infections. Other clinicians have suggested that neonates may receive ceftizoxime in a dosage of 25-50 mg/kg every 12 hours.
Dosage in Renal Impairment
In patients with impaired renal function, doses and/or frequency of administration of ceftizoxime must be modified in response to the degree of renal impairment, severity of the infection, susceptibility of the causative organism, and serum concentrations of the drug.
The manufacturer recommends that adults with impaired renal function receive an initial loading dose of 500 mg to 1 g IM or IV and the following maintenance dosage based on the patient’s creatinine clearance:1 Creatinine Clearance Life-Threatening Less Severe Infections (mL/min) Infections 50-79 750 mg to 1.5 g every 8 h 500 mg every 8 h 5-49 500 mg to 1 g every 12 h 250-500 mg every 12 h <5 500 mg to 1 g every 48 h 500 mg every 48 h or or 500 mg every 24 h 250 mg every 24 h In patients undergoing hemodialysis, the manufacturer states that supplemental doses of ceftizoxime are unnecessary following hemodialysis but that the dosing regimen should be timed so that a dose of ceftizoxime is scheduled at the end of the dialysis period.
Cautions
Adverse effects reported with ceftizoxime are similar to those reported with other cephalosporins.
Dermatologic Reactions and Sensitivity
Hypersensitivity reactions, including rash, pruritus, and fever, have been reported in less than 5% of patients receiving ceftizoxime. Numbness, which may be a hypersensitivity reaction, and anaphylactoid-type reactions have been reported rarely. Positive direct antiglobulin (Coombs’) test results have been reported in a few patients receiving the drug; however, it is not clear whether the mechanism of this reaction is immunologic in nature. If a severe hypersensitivity reaction occurs during ceftizoxime therapy, the drug should be discontinued and the patient given appropriate therapy (e.g., epinephrine, corticosteroids, IV fluids, IV antihistamines, vasopressors, maintenance of an adequate airway, oxygen) as indicated. Erythema multiforme (e.g., Stevens-Johnson syndrome) toxic epidermal necrolysis, and serum sickness-like reactions have been reported in patients receiving cephalosporins.
Local Effects
Adverse effects at the injection site have been reported in less than 5% of patients receiving ceftizoxime. Burning, cellulitis, pain, induration, tenderness, paresthesia, and phlebitis have been reported.
Hematologic Effects
Transient eosinophilia or thrombocytosis has been reported in less than 5% of patients receiving ceftizoxime. Neutropenia, leukopenia, thrombocytopenia, and anemia have been reported in less than 1% of patients receiving the drug. Prolongation of the prothrombin time and hypoprothrombinemia have been reported rarely in patients receiving ceftizoxime. Immune-mediated hemolytic anemia with extravascular hemolysis has been reported in at least 2 patients receiving ceftizoxime. Similar cases, including some fatalities, have been reported rarely with ceftriaxone, cefotaxime, and cefotetan. Aplastic anemia, hemorrhage, pancytopenia, and agranulocytosis have been reported in patients receiving cephalosporins.
Hepatic Effects
Transient increases in serum AST (SGOT), ALT (SGPT), and alkaline phosphatase concentrations have been reported in less than 5% of patients receiving ceftizoxime. Increased serum bilirubin has been reported rarely. Increased serum LDH concentrations have been reported in patients receiving cephalosporins.
GI Effects
Adverse GI effects, including diarrhea, nausea, and vomiting, have been reported occasionally with ceftizoxime. Clostridium difficile-associated diarrhea and colitis (also known as antibiotic-associated pseudomembranous colitis), caused by toxin-producing clostridia resistant to ceftizoxime, can occur during or following discontinuance of ceftizoxime therapy. Mild cases of colitis may respond to discontinuance of ceftizoxime alone, but diagnosis and management of moderate to severe cases should include appropriate bacteriologic studies and treatment with fluid, electrolyte, and protein supplementation as indicated; rarely, cautious use of sigmoidoscopy (or other appropriate endoscopic examination) may be considered necessary. If colitis is moderate to severe or is not relieved by discontinuance of ceftizoxime, appropriate anti-infective therapy (e.g., oral metronidazole or vancomycin) should be administered. Isolation of the patient may be advisable.Other causes of colitis also should be considered.
Renal Effects
Transient increases in BUN and serum creatinine concentrations have been reported occasionally with ceftizoxime. Toxic nephropathy has been reported in patients receiving cephalosporins.
Other Adverse Effects
Headache, dizziness, and tinnitus have been reported rarely with ceftizoxime. Vaginitis has also occurred rarely in patients receiving the drug. Several cephalosporins have been associated with seizures, generally when usual dosages of the drugs were used in patients with renal impairment. If seizures do occur during ceftizoxime therapy, the drug should be discontinued and appropriate anticonvulsant therapy administered as indicated.
Precautions and Contraindications
Prior to initiation of ceftizoxime therapy, careful inquiry should be made concerning previous hypersensitivity reactions to cephalosporins, penicillins, or other drugs. There is clinical and laboratory evidence of partial cross-allergenicity among cephalosporins and other b-lactam antibiotics including penicillins and cephamycins; however, the true incidence of cross-allergenicity among these anti-infectives has not been established.
Ceftizoxime is contraindicated in patients who are hypersensitive to the drug or other cephalosporins and should be used with caution in patients with a history of hypersensitivity to penicillins. Use of cephalosporins should be avoided in patients who have had an immediate-type (anaphylactic) hypersensitivity reaction to penicillins.
Although it has not been definitely proven that allergic reactions to antibiotics are more frequent in atopic individuals, the manufacturer states that ceftizoxime should be used with caution in patients with a history of allergy, particularly to drugs.
Prolonged use of ceftizoxime may result in overgrowth of nonsusceptible organisms. Careful observation of the patient during ceftizoxime therapy is essential. If suprainfection or superinfection occurs, appropriate therapy should be instituted.
Ceftizoxime should be used with caution in patients with a history of GI disease, particularly colitis. Because C. difficile-associated diarrhea and colitis has been reported with the use of cephalosporins, it should be considered in the differential diagnosis of patients who develop diarrhea during ceftizoxime therapy. Although ceftizoxime has only rarely caused adverse renal effects, the manufacturer states that renal function should be monitored during therapy with the drug, especially when maximum dosage is used in seriously ill patients.
Because serum concentrations of ceftizoxime are higher and more prolonged in patients with renal impairment than in patients with normal renal function, dose and/or frequency of administration of the drug should be decreased in patients with impaired renal function. (See Dosage and Administration: Dosage in Renal Impairment.)
Pediatric Precautions
The manufacturer states that safety and efficacy of ceftizoxime in neonates and infants younger than 6 months of age have not been established. In children 6 months of age and older, use of ceftizoxime has been associated with transient increases in eosinophil counts and in serum AST, ALT, and creatine kinase (CK, creatine phosphokinase, CPK) concentrations. Increases in serum CK (CPK) concentrations may have been related to IM injection of the drug. Safety of the chemical components that may leach out of the plastic containing commercially available frozen ceftizoxime injections has not been established in children.
Mutagenicity and Carcinogenicity
No evidence of mutagenicity was seen when ceftizoxime was evaluated in several in vitro and in vivo test systems. At concentrations of 0.001-0. mcg per plate, ceftizoxime was not mutagenic in the Ames microbial test. There also was no evidence of mutagenicity in micronucleus assays in mice using ceftizoxime doses up to 7500 mg/kg (approximately 6 times the maximum human dose). Long-term animal carcinogenicity studies using ceftizoxime have not been conducted to date.
Pregnancy, Fertitlity and Lactation
Reproduction studies in rats and rabbits using ceftizoxime have not revealed evidence of impaired fertility or harm to the fetus. Reproduction studies in rats using subcutaneous ceftizoxime in dosages up to 1000 mg/kg daily (approximately 2 times the maximum human dosage) have not revealed evidence of impaired fertility. In addition, there has been no evidence of histologic changes in the reproductive organs of male and female dogs receiving IV ceftizoxime in dosages of 1000 mg/kg daily (approximately 5 times the maximum human dosage) for 13 weeks. There are no adequate and controlled studies to date using ceftizoxime in pregnant women, and the drug should be used during pregnancy only when clearly needed. The manufacturer states that safety of ceftizoxime during labor and delivery has not been established. Because ceftizoxime is distributed into milk, the drug should be used with caution in nursing women.
Drug Interactions
Probenecid
Oral probenecid administered shortly before or concomitantly with ceftizoxime slows the rate of renal tubular secretion of ceftizoxime and produces higher and more prolonged serum concentrations of the drug.
Aminoglycosides
Concurrent use of aminoglycosides and certain cephalosporins reportedly may increase the risk of nephrotoxicity during therapy. Although this effect has not been reported to date with ceftizoxime, the manufacturer states that the possibility that nephrotoxicity may be potentiated should be considered if the drug is used concomitantly with an aminoglycoside and renal function should be monitored.
Laboratory Test Interferences
Immunohematology Tests
Positive direct antiglobulin (Coombs’) test results have been reported in a few patients receiving ceftizoxime. This action may interfere with hematologic studies or transfusion cross-matching procedures.
Tests for Creatinine
At concentrations greater than 250 mcg/mL, ceftizoxime may cause falsely elevated serum or urine creatinine values when the Jaffe reaction is used.
Tests for Urinary Glucose
It is not known if ceftizoxime, like most other cephalosporins, causes false-positive results in urine glucose determinations using cupric sulfate solution (Benedict’s reagent, Clinitest®).
Mechanism of Action
Ceftizoxime usually is bactericidal in action. Like other cephalosporins, the antibacterial activity of the drug results from inhibition of mucopeptide synthesis in the bacterial cell wall. For information on the mechanism of action of cephalosporins, see Mechanism of Action in the Cephalosporins General Statement 8:12.06. Spectrum Based on its spectrum of activity, ceftizoxime is classified as a third generation cephalosporin.
For information on the classification of cephalosporins and closely related b-lactam antibiotics based on spectra of activity, see Spectrum in the Cephalosporins General Statement 8:12.06. Like other currently available parenteral third generation cephalosporins (e.g., cefoperazone, cefotaxime, ceftazidime, ceftriaxone), ceftizoxime generally is less active in vitro against susceptible staphylococci than first generation cephalosporins but has an expanded spectrum of activity against gram-negative bacteria compared with first and second generation cephalosporins.
The spectrum of activity of ceftizoxime resembles that of other parenteral third generation cephalosporins. In vitro on a weight basis, the activity of ceftizoxime against susceptible Enterobacteriaceae is approximately equal to that of cefotaxime. However, in vitro on a weight basis, ceftizoxime is generally more active than cefoperazone, cefotaxime, or ceftriaxone against most strains of Serratia.
In Vitro Susceptibility Testing
Results of in vitro ceftizoxime susceptibility tests are not generally affected by inoculum size, culture media, presence of serum, or pH. However, results of susceptibility tests for some gram-negative bacilli (e.g., Bacteroides fragilis, Escherichia coli, Pseudomonas aeruginosa, Serratia) may be affected by the size of the inoculum.
The National Committee for Clinical Laboratory Standards (NCCLS) states that, if results of in vitro susceptibility testing indicate that a clinical isolate is susceptible to ceftizoxime, then an infection caused by this strain may be appropriately treated with the dosage of the drug recommended for that type of infection and infecting species, unless otherwise contraindicated.
If results indicate that a clinical isolate has intermediate susceptibility to ceftizoxime, then the strain has a minimum inhibitory concentration (MIC) that approaches usually attainable blood and tissue drug concentrations and response rates may be lower than for strains identified as susceptible. Therefore, the intermediate category implies clinical applicability in body sites where the drug is physiologically concentrated (e.g., urine) or when a high dosage of the drug can be used.
This intermediate category also includes a buffer zone which should prevent small, uncontrolled technical factors from causing major discrepancies in interpretation, especially for drugs with narrow pharmacotoxicity margins. If results of in vitro susceptibility testing indicate that a clinical isolate is resistant to ceftizoxime, the strain is not inhibited by systemic concentrations of the drug achievable with usual dosage schedules and/or MICs fall in the range where specific microbial resistance mechanisms are likely and efficacy has not been reliably demonstrated in clinical trials. Strains of staphylococci resistant to penicillinase-resistant penicillins also should be considered resistant to ceftizoxime, although results of in vitro susceptibility tests may indicate that the organisms are susceptible to the drug.
Disk Susceptibility Tests
When the disk-diffusion procedure is used to test susceptibility to ceftizoxime, a disk containing 30 mcg of ceftizoxime should be used. The cephalosporin class disk containing 30 mcg of cephalothin or disks containing other cephalosporins should not be used for testing susceptibility to ceftizoxime. When disk-diffusion susceptibility testing is performed according to NCCLS standardized procedures using NCCLS interpretive criteria, Staphylococcus, Enterobacteriaceae, or urinary isolates of Pseudomonas aeruginosa or Acinetobacter with growth inhibition zones of 20 mm or greater are susceptible to ceftizoxime, those with zones of 15-19 mm have intermediate susceptibility, and those with zones of 14 mm or less are resistant to the drug. When disk-diffusion susceptibility testing is performed according to NCCLS standardized procedures using Haemophilus test medium (HTM), Haemophilus with growth inhibition zones of 26 mm or greater are susceptible to ceftizoxime.
Because of limited data on resistant strains of these organisms, NCCLS recommends that any Haemophilus isolate that appears to be nonsusceptible to ceftizoxime be submitted to a reference laboratory for further testing. When disk-diffusion susceptibility testing is performed according to the NCCLS standardized procedures using GC agar (with 1% defined growth supplement), N. gonorrhoeae with growth inhibition zones of 38 mm or greater are susceptible to ceftizoxime. Because of limited data on resistant strains of these organisms, NCCLS recommends that any N. gonorrhoeae isolate that appears to be nonsusceptible to ceftizoxime be submitted to a reference laboratory for further testing. Interpretive criteria are not available to determine susceptibility of streptococci to ceftizoxime using the ceftizoxime disk; however, NCCLS states that S. pneumoniae found to be susceptible to penicillin using the NCCLS standardized disk-diffusion procedure and a 1-mcg oxacillin disk can be considered susceptible to ceftizoxime. In addition, other streptococci (b-hemolytic streptococci, viridans streptococci) found to be susceptible to penicillin can be considered susceptible to ceftizoxime.
Dilution Susceptibility Tests
When dilution susceptibility testing (agar or broth dilution) is performed according to NCCLS standardized procedures using NCCLS interpretive criteria, Staphylococcus, Enterobacteriaceae, and urinary isolates of Ps. aeruginosa and other non-Enterobacteriaceae gram-negative bacilli (e.g., other Pseudomonas spp., Acinetobacter, Stenotrophomonas maltophilia) with MICs of 8 mcg/mL or less are susceptible to ceftizoxime, those with MICs of 16-32 mcg/mL have intermediate susceptibility, and those with MICs of 64 mcg/mL or greater are resistant to the drug.
When dilution susceptibility testing for Haemophilus is performed according to NCCLS standardized procedures using HTM, isolates with MICs of 2 mcg/mL or less are susceptible to ceftizoxime.
Because of limited data on resistant strains of these organisms, NCCLS recommends that any Haemophilus isolate that appears to be nonsusceptible to ceftizoxime be submitted to a reference laboratory for further testing. When dilution susceptibility testing for N. gonorrhoeae is performed according to the NCCLS standardized procedure using GC agar base (with 1% defined growth supplement), isolates with MICs of 0.5 mcg/mL or less are susceptible to ceftizoxime. Because of limited data on resistant strains of these organisms, NCCLS recommends that any Neisseria isolate that appears to be nonsusceptible to ceftizoxime be submitted to a reference laboratory for further testing. Interpretive criteria are not available for broth dilution susceptibility testing of streptococci using ceftizoxime; however, NCCLS states that S. pneumoniae and other streptococci (b-hemolytic streptococci, viridans streptococci) found to be susceptible to penicillin using NCCLS standardized procedures can be considered susceptible to ceftizoxime.
Gram-positive Aerobic Bacteria
In vitro, ceftizoxime concentrations of 4 mcg/mL inhibit most strains of Staphylococcus aureus and many strains of S. epidermidis. Ceftizoxime is active in vitro against most penicillinase-producing staphylococci; however, staphylococci resistant to penicillinase-resistant penicillins also are resistant to ceftizoxime. The MIC90 (minimum inhibitory concentration of the drug at which 90% of strains tested are inhibited) of ceftizoxime reported for groups A and B streptococci and Streptococcus pneumoniae is 0.2 mcg/mL2 and for viridans streptococci is 0.4 mcg/mL.
Enterococci, including E. faecalis (formerly S. faecalis), are generally resistant to ceftizoxime. Ceftizoxime also is active in vitro against Corynebacterium diphtheriae.
Although some strains of Listeria monocytogenes are reportedly inhibited in vitro by ceftizoxime concentrations of 12. mcg/mL, most strains of the organism are considered resistant to the drug.
Gram-negative Aerobic Bacteria
Generally, ceftizoxime is active in vitro against the following Enterobacteriaceae: Citrobacter freundii, Enterobacter aerogenes, E. cloacae, Escherichia coli, Klebsiella pneumoniae, Morganella morganii (formerly Proteus morganii), Proteus mirabilis, P. vulgaris, Providencia, Salmonella, Serratia marcescens, and Shigella. In one study, the MIC90 of ceftizoxime for most of these Enterobacteriaceae was 1.6 mcg/mL or less; however, the MIC90 for S. marcescens was 12. mcg/mL. Ceftizoxime concentrations of 6.3 mcg/mL inhibit some strains of S. marcescens resistant to gentamicin and tobramycin. Although some strains of Ps. aeruginosa are inhibited in vitro by ceftizoxime concentrations of 0.1-32 mcg/mL, most strains of this organism are considered resistant to the drug. Ceftizoxime is less active in vitro against susceptible Ps. aeruginosa than is cefoperazone, ceftazidime, or some extended-spectrum penicillins.
Ceftizoxime is active in vitro against Haemophilus influenzae (including ampicillin-resistant strains), Neisseria gonorrhoeae (including penicillinase-producing strains), and N. meningitidis. The MIC90 of the drug for these gram-negative bacteria is reportedly 0.03-1. mcg/mL. Ceftizoxime is active in vitro against b-lactamase- and non-b-lactamase-producing strains of Moraxella (formerly Branhamella) catarrhalis, and the MIC90 of the drug for this organism is 0.1-0. mcg/mL. Some strains of Acinetobacter are inhibited in vitro by ceftizoxime concentrations of 0.05-25 mcg/mL. Eikenella corrodens generally is inhibited in vitro by ceftizoxime concentrations of 0.03-16 mcg/mL, and the MIC90 of the drug for this organism is 4 mcg/mL. Ceftizoxime also is active in vitro against Aeromonas hydrophila, Yersinia enterocolitica, and Pasteurella multocida.
Anaerobic Bacteria
Ceftizoxime is active in vitro against many anaerobic bacteria including Actinomyces, B. fragilis, Bifidobacterium, Eubacterium, Fusobacterium, Peptococcus, Peptostreptococcus, Propionibacterium, and Veillonella. Ceftizoxime also is active in vitro against some strains of Clostridium including C. perfringens; however, C. difficile usually is resistant to the drug. Susceptible anaerobic bacteria generally are inhibited in vitro by ceftizoxime concentrations of 16 mcg/mL or less. Some strains of B. fragilis, B. vulgatus, or B. distasonis are inhibited in vitro by ceftizoxime concentrations of 2.1-25 mcg/mL; however the MIC90 of the drug for B. fragilis or B. vulgatus ranges from 8-128 mcg/mL. The MIC90 of ceftizoxime for B. distasonis, B. thetaiotamicron, B. ovatus, or B. uniformis is 32-128 mcg/mL or greater. Resistance For information on possible mechanisms of bacterial resistance to cephalosporins, see Resistance in the Cephalosporins General Statement 8:12.06.
Ceftizoxime generally is more resistant to inactivation by b-lactamases than is cefoperazone, cefamandole, or cefuroxime but less resistant to inactivation by b-lactamases than cefotaxime or cefoxitin. Ceftizoxime generally is resistant to inactivation by b-lactamases that act principally as penicillinases and those which act principally as cephalosporinases. The drug generally is not hydrolyzed by b-lactamases classified as Richmond-Sykes types I, II, III (TEM types), and IV, or by some enzymes produced by Ps. aeruginosa or S. aureus.
Pharmacokinetics
In all studies described in the Pharmacokinetics section, ceftizoxime was administered as the sodium salt; dosages and concentrations of the drug are expressed in terms of ceftizoxime.
Absorption
Ceftizoxime sodium is not appreciably absorbed from the GI tract and must be given parenterally. Following IM administration of a single 500-mg or 1-g dose of ceftizoxime in healthy adults, peak serum concentrations of the drug are attained within 0.5-1. hours and average 13. mcg/mL and 39-40. mcg/mL, respectively. In one study following IM administration of a single 1-g dose in healthy adults, serum ceftizoxime concentrations averaged 39 mcg/mL 1 hour after the dose, 15 mcg/mL 4 hours after the dose, and 3 mcg/mL 8 hours after the dose
. Following IV administration over 30 minutes of a single 1-g dose of ceftizoxime in healthy adults, serum concentrations of the drug average 84. mcg/mL at the end of the infusion, 41. mcg/mL at 1 hour, 16. mcg/mL at 2 hours, 6.4 mcg/mL at 4 hours, and 2.1 mcg/mL at 7 hours after the start of the infusion.
Following IV injection of a single 1-g dose of the drug in healthy adults, serum concentrations of the drug average 60. mcg/mL at 30 minutes, 21. mcg/mL at 2 hours, 8.4 mcg/mL at 4 hours, and 1.4 mcg/mL at 8 hours after the dose. IV injection of a single 2- or 3-g dose of the drug in healthy adults results in serum concentrations that average 131. or 221. mcg/mL at 5 minutes, 77. or 112. mcg/mL at 30 minutes, 33. or 47. mcg/mL at 2 hours, 12. or 26. mcg/mL at 4 hours, and 2 or 4.8 mcg/mL at 8 hours, respectively, after the dose.
Following IV administration over 15-30 minutes of a single 25- or 50-mg/kg dose of ceftizoxime in neonates and infants 6 months of age or younger, plasma ceftizoxime concentrations average 64 or 118 mcg/mL, respectively, 30 minutes after the dose and 22 or 30 mcg/mL, respectively, 8 hours after the dose.
Distribution
Following IM or IV administration, ceftizoxime is widely distributed into body tissues and fluids including the heart, gallbladder, bone, prostatic tissue, uterine tissue, biliary tissue, ascitic fluid, peritoneal fluid, pleural fluid, surgical wound fluid, aqueous humor, bile, and saliva. Ceftizoxime also is distributed into CSF if meninges are inflamed.
Following IV infusion over 20 minutes of ceftizoxime 30 mg/kg in patients with inflamed meninges, CSF concentrations of the drug 45 minutes to 3.3 hours after the dose reportedly average 4.6 mcg/mL. In one study in patients with inflamed meninges receiving ceftizoxime in a dosage of 30 mg/kg IV over 5-30 minutes every 4 hours, CSF concentrations ranged from undetectable to 29 mcg/mL in samples obtained 30 minutes to 7 hours after dosing.
The apparent volume of distribution of ceftizoxime in adults is 0.29-0. L/kg. In neonates and infants 6 months of age or younger, the steady-state volume of distribution of ceftizoxime averages 0.36-0. L/kg. Ceftizoxime is 28-31% bound to serum proteins. Ceftizoxime crosses the placenta and is distributed into milk.
Elimination
The serum half-life of ceftizoxime in adults with normal renal function ranges from 1.4-1. hours. In patients with renal impairment, serum concentrations of ceftizoxime are higher and the serum half-life of the drug is prolonged. In one study, the half-life of ceftizoxime was 3.85 hours in patients with creatinine clearances of 30.-47. mL/minute, 20. hours in patients with creatinine clearances of 6.8-13. mL/minute, and 30. hours in patients with creatinine clearances of 0-1. mL/minute.
The serum half-life of ceftizoxime is longer in neonates than in older children and adults with normal renal function. In one study in neonates and children 6 months of age or younger, the plasma half-life of ceftizoxime averaged 7.2 hours in neonates 1 day of age or younger, 6.3 or 4.7 hours in those 2-7 or 8-30 days of age, respectively, and 2.4 hours in those older than 30 days of age. Ceftizoxime is not metabolized and is excreted principally in urine by both glomerular filtration and tubular secretion. In adults with normal renal function, 58-92% of a single 500-mg or 1-g IM or IV dose of the drug is excreted in urine unchanged within 24 hours.
Following IM administration of a single 500-mg dose of ceftizoxime, urinary concentrations of the drug range from 700 mcg/mL to 3.2 mg/mL in urine collected during the first 4 hours after administration and 8-130 mcg/mL in urine collected 8-24 hours after administration.
Urinary concentrations of ceftizoxime greater than 6 mg/mL have been reported in urine collected during the first 2 hours following IV administration of a single 1-g dose of the drug. Concomitant administration of probenecid inhibits renal tubular secretion of ceftizoxime and produces higher and more prolonged serum concentrations of ceftizoxime. Ceftizoxime is removed to a limited extent by hemodialysis and by peritoneal dialysis.
Chemistry and Stability
Chemistry
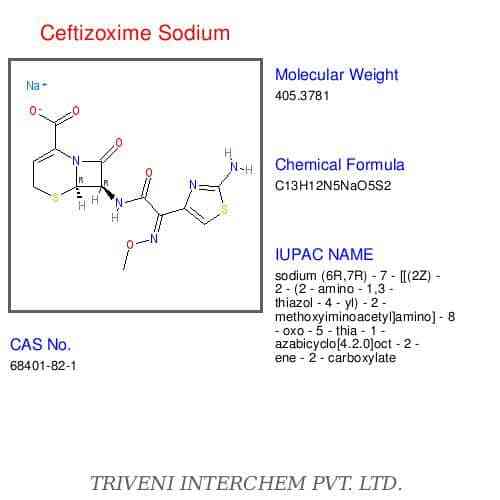
Ceftizoxime is a semisynthetic cephalosporin antibiotic. Ceftizoxime is the desacetoxymethyl derivative of cefotaxime. Like cefepime, ceftazidime, cefotaxime, and ceftriaxone, ceftizoxime is a parenteral aminothiazolyl cephalosporin.
Ceftizoxime contains an aminothiazolyl-acetyl side chain, with a methoxyimino group, at position 7 on the cephalosporin nucleus. The aminothiazolyl side chain enhances antibacterial activity, particularly against Enterobacteriaceae, and generally results in enhanced stability against b-lactamases; the methoxyimino group contributes to stability against hydrolysis by many b-lactamases. Ceftizoxime is commercially available as the sodium salt.
Potency of ceftizoxime sodium is expressed in terms of ceftizoxime. Ceftizoxime sodium occurs as a white to pale yellow, crystalline powder. The drug has a solubility of 500 mg/mL in water at 25°C19 and pKas of 2.1 and 2.7. The sodium salt of ceftizoxime contains 2.6 mEq of sodium per gram of ceftizoxime. Reconstituted solutions of ceftizoxime sodium are generally colorless to pale yellow in color and have a pH of 6-8.A solution of 1 g of ceftizoxime per 13 mL of sterile water for injection is isotonic.
The commercially available frozen ceftizoxime sodium injections containing 1 or 2 g of ceftizoxime in 50 mL of iso-osmotic dextrose injection (3. or 1.9%, respectively) are nonpyrogenic, sterile solutions of the drug; these solutions have a pH of 5.5-8.
Stability
Commercially available ceftizoxime sodium powder for injection should be stored at 15-30°C and protected from excessive light. The commercially available frozen ceftizoxime sodium injection should be stored at a temperature not greater than -20°C.
Reconstituted solutions of ceftizoxime sodium may darken depending on storage conditions. Although reconstituted solutions are initially clear to pale yellow, a color change ranging from yellow to amber does not indicate loss of potency. Ceftizoxime sodium solutions should be discarded if a precipitate forms and if particulate matter is present in the solution.
Following reconstitution with sterile water for injection, ceftizoxime sodium solutions containing 95 mg of ceftizoxime per mL are stable for 24 hours at room temperature or 96 hours at 5°C and solutions containing 270 or 280 mg of ceftizoxime per mL are stable for 16 hours at room temperature.
The manufacturer states that the stability of the commercially available frozen ceftizoxime sodium injection may vary. These injections are stable for at least 90 days from the date of shipment when stored at -20°C. The frozen injection should be thawed at room temperature and, once thawed, should not be refrozen. Thawed solutions of the commercially available frozen injection are stable for 48 hours at room temperature (25°C) or 21 days when refrigerated at 5°C.
The commercially available frozen injection of the drug is provided in a plastic container fabricated from specially formulated polyvinyl chloride (PVC). Solutions in contact with the plastic can leach out some of its chemical components in very small amounts (e.g., bis(2-ethylhexyl)phthalate [BEHP, DEHP] in up to 5 ppm) within the expiration period of the injection; however, safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies. Ceftizoxime sodium is physically and chemically compatible with the following IV solutions: 0.9% sodium chloride; 5 or 10% dextrose; 5% dextrose and 0.2, 0.45, or 0.9% sodium chloride; Ringer’s; lactated Ringer’s; 10% invert sugar; or 5% sodium bicarbonate. If ceftizoxime sodium is initially reconstituted with 4% sodium bicarbonate injection, the drug is also compatible with 5% dextrose in lactated Ringer’s injection.
Reconstituted solutions of ceftizoxime sodium that have been further diluted with 50-100 mL of one of the above IV solutions are stable for 24 hours at room temperature or 96 hours at 5°C. Ceftizoxime sodium is potentially physically and/or chemically incompatible with some drugs, including aminoglycosides, but the compatibility depends on several factors (e.g., concentrations of the drugs, specific diluents used, resulting pH, temperature).
Specialized references should be consulted for specific compatibility information. For further information on chemistry, mechanism of action, spectrum, resistance, pharmacokinetics, uses, cautions, drug interactions, laboratory test interferences, and dosage and administration of ceftizoxime, see the Cephalosporins General Statement 8:12.06
Preparations
Ceftizoxime Sodium Parenteral For injection 500 mg (of ceftizoxime) Cefizox®, Fujisawa 1 g (of ceftizoxime) Cefizox®, Fujisawa 2 g (of ceftizoxime) Cefizox®, Fujisawa 10 g (pharmacy bulk package) Cefizox®, Fujisawa For injection, for 1 g (of ceftizoxime) Cefizox® ADD-Vantage®, IV infusion Fujisawa Cefizox® Piggyback, Fujisawa 2 g (of ceftizoxime) Cefizox® ADD-Vantage®, Fujisawa Cefizox® Piggyback, Fujisawa Ceftizoxime Sodium in Dextrose Parenteral Injection (frozen) 20 mg (of ceftizoxime) per Cefizox® in Iso-osmotic , for IV infusion mL (1 g) in 3.8% Dextrose Dextrose Injection, (Galaxy® [Baxter]) Fujisawa 40 mg (of ceftizoxime) per Cefizox® in Iso-osmotic mL (2 g) in 1.9% Dextrose Dextrose Injection, (Galaxy® [Baxter]) Fujisawa

