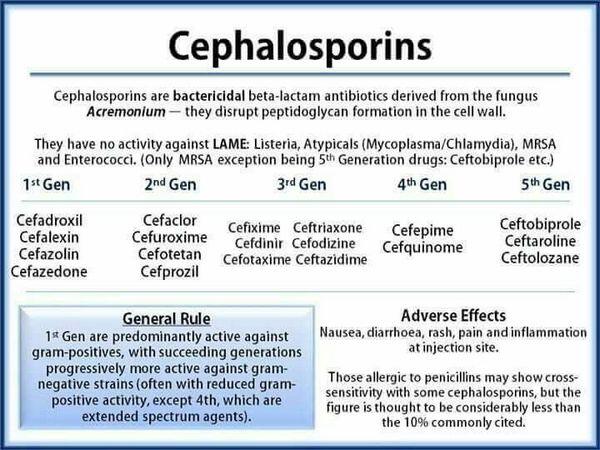
Cephalosporins are semisynthetic b-lactam antibiotics that are structurally and pharmacologically related to penicillins, carbacephems (e.g., loracarbef), and cephamycins (e.g., cefotetan, cefoxitin). Cephalosporins generally are divided into 4 groups (“generations”) based on their spectra of activity.
Uses
Cephalosporins are used parenterally for the treatment of lower respiratory tract, skin and skin structure, urinary tract, and bone and joint infections caused by susceptible gram-positive or gram-negative bacteria and also are used parenterally for the treatment of meningitis and septicemia/bacteremia caused by susceptible gram-positive or gram-negative bacteria.
Cephalosporins also are used parenterally for the treatment of intra-abdominal, biliary tract, and gynecologic infections (including pelvic inflammatory disease) caused by susceptible bacteria.
Cefotaxime, cefoxitin, ceftizoxime, ceftriaxone, and cefuroxime are used parenterally for the treatment of uncomplicated gonorrhea or other gonococcal infections; cefepime, ceftazidime, and ceftriaxone are used for empiric anti-infective therapy in febrile neutropenic patients; and cefazolin, cefotaxime, ceftriaxone, and cefuroxime are used parenterally for perioperative prophylaxis.
Cephalosporins are used orally for the treatment of mild to moderate respiratory tract infections, including acute maxillary sinusitis, acute bacterial exacerbations of chronic bronchitis, secondary infections of acute bronchitis, and community-acquired pneumonia, caused by susceptible bacteria (e.g., Streptococcus pneumoniae, Haemophilus influenzae, H. parainfluenzae, Moraxella catarrhalis [formerly Branhamella catarrhalis]); acute bacterial otitis media caused by susceptible bacteria (e.g., S. pneumoniae, H. influenzae, M. catarrhalis); and pharyngitis and tonsillitis caused by Streptococcus pyogenes (group A b-hemolytic streptococci).
Cefaclor, cefadroxil, cefdinir, cefditoren pivoxil, cefpodoxime proxetil, cefprozil, ceftibuten, cefuroxime axetil, cephalexin, and cephradine also are used orally for the treatment of mild to moderate skin and skin structure infections caused by susceptible staphylococci or streptococci. In addition, cefaclor, cefadroxil, cefixime, cefpodoxime proxetil, ceftibuten, cefuroxime axetil, cephalexin, and cephradine are used orally for the treatment of mild to moderate urinary tract infections caused by susceptible gram-negative bacteria (e.g., Escherichia coli, Klebsiella, Proteus mirabilis).
Some clinicians suggest that certain oral third generation cephalosporins (cefdinir, cefditoren pivoxil, cefixime, cefpodoxime proxetil, ceftibuten) are one of several alternatives that can be used for the outpatient treatment of recurrent urinary tract infections or urinary tract infections acquired in hospitals or nursing homes since these infections are likely to be caused by multidrug-resistant gram-negative bacilli; however, these oral cephalosporins are not appropriate for the treatment of more severely ill patients hospitalized with urinary tract infections.
Although not considered drugs of choice, certain oral cephalosporins (e.g., cefixime, cefuroxime axetil, cefpodoxime proxetil) are used for the treatment of uncomplicated gonorrhea. In addition, oral cefixime is used in the treatment of disseminated gonococcal infections. (See Uses: Gonorrhea and Associated Infections. Prior to and during cephalosporin therapy, the causative organism should be cultured and in vitro susceptibility tests performed. In serious infections, therapy may be initiated pending results of in vitro tests. In certain serious infections when the causative organism is unknown, concomitant therapy with another anti-infective agent (e.g., an aminoglycoside) may be indicated pending results of susceptibility tests. Use of a cephalosporin does not replace surgical procedures such as incision and drainage when indicated.
Gram-positive Bacterial Infections
Third generation cephalosporins generally are less active than first and second generation cephalosporins against gram-positive bacteria, especially staphylococci, and usually are not used in the treatment of infections caused by gram-positive bacteria when a penicillin or a first or second generation cephalosporin could be used. However, some third generation cephalosporins (e.g., cefotaxime, ceftriaxone) are considered drugs of choice for serious bacterial infections, including endocarditis or meningitis, caused by susceptible S. pneumoniae or viridans streptococci.
Gram-negative Bacterial Infections
Use of first generation cephalosporins (cefazolin, cephradine) in the treatment of gram-negative bacterial infections generally is limited to infections caused by susceptible E. coli, H. influenzae, Klebsiella, or P. mirabilis. Second and third generation cephalosporins are used in the treatment of infections caused by these organisms as well as infections caused by susceptible Enterobacter, Morganella morganii (formerly Proteus morganii), Neisseria, Providencia rettgeri (formerly P. rettgeri), or P. vulgaris; cefotaxime, ceftazidime, ceftizoxime, and ceftriaxone also are used in the treatment of infections caused by susceptible Serratia.
Certain parenteral third generation cephalosporins (i.e., cefepime, cefotaxime, ceftazidime, ceftizoxime, ceftriaxone) may be drugs of choice for the treatment of infections caused by susceptible Enterobacteriaceae, including susceptible strains of E. coli, K. pneumoniae, P. rettgeri, M. morganii, P. vulgaris, or P. stuartii and are alternatives for the treatment of infections caused by susceptible Serratia; an aminoglycoside usually is used concomitantly in severe infections.
Ceftazidime (but not cefotaxime, ceftizoxime, or ceftriaxone) is considered a drug of choice for the treatment of infections caused by susceptible Pseudomonas aeruginosa; an aminoglycoside may be used concomitantly.
Ceftazidime is more active in vitro on a weight basis against Ps. aeruginosa than most other currently available cephalosporins and is active against some strains resistant to many other cephalosporins, aminoglycosides, and extended-spectrum penicillins.
Otitis Media
Acute Otitis Media
Various oral cephalosporins (e.g., cefaclor, cefdinir, cefixime, cefpodoxime proxetil, cefprozil, ceftibuten, cefuroxime axetil, cephalexin, cephradine) are used for the treatment of acute otitis media (AOM) caused by Streptococcus pneumoniae, Haemophilus influenzae (including b-lactamase-producing strains), or Moraxella catarrhalis (including b-lactamase-producing strains).
IM ceftriaxone also is used for the treatment of AOM caused by S. pneumoniae, H. influenzae (including b-lactamase-producing strains), or M. catarrhalis (including b-lactamase-producing strains).
AOM is the most frequently diagnosed bacterial infection in children, and 65-95% of children will have at least one episode of AOM by 3 years of age. AOM is defined as the presence of fluid in the middle ear accompanied by signs or symptoms of acute local or systemic illness (e.g., otalgia, otorrhea, hearing loss, swelling around the ear, vertigo, nystagmus, tinnitus, fever, irritability, headache, diarrhea, lethargy, anorexia, vomiting).
Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis are the bacteria most frequently recovered from middle ear fluid of patients with AOM; S. pyogenes and S. aureus also are recovered rarely. In addition, there is evidence that respiratory viruses (e.g., respiratory syncytial virus, rhinoviruses, influenza virus, parainfluenza virus, enteroviruses) may be present either alone or in combination with bacterial pathogens and may play a role in the etiology and pathogenesis of AOM in some patients.
Management and Therapy Considerations
Up to 60-80% of cases of AOM resolve spontaneously within 7-14 days, and some clinicians have questioned the necessity of routine administration of anti-infectives for the treatment of all cases of AOM.
These clinicians suggest that, for the majority of patients with uncomplicated AOM, anti-infective therapy appears to provide only minimal benefits in terms of resolution of the acute symptoms of infection (e.g., pain) and the proposed benefits of such therapy in terms of time to bacteriologic or clinical resolution of AOM or in terms of long-term consequences of otitis media (e.g., persistence of middle ear effusion, recurrence of AOM, hearing loss, need for adenoidectomy or insertion of tympanostomy tubes, mastoiditis) have never been substantiated in well-designed, placebo-controlled studies. In addition, there is evidence that overuse of anti-infectives, including overuse in the treatment of AOM, contributes to emergence of resistant bacteria (e.g., multidrug-resistant S. pneumoniae).
These clinicians suggest a management strategy for AOM that involves use of symptomatic care with analgesics and close observation via telephone contact or office visits for the majority of patients with uncomplicated AOM and use of anti-infectives only in those who do not have symptomatic improvement within 24-48 hours of diagnosis and in those who appear least likely to have spontaneous resolution and most likely to have poor outcomes (e.g., more acutely ill, those with 3 or more episodes of AOM in the past 18 months, history of serous otitis or tympanostomy tubes).
However, the American Academy of Pediatrics (AAP), the US Centers for Disease Control and Prevention (CDC), and other clinicians currently recommend that all cases of AOM be treated with an appropriate anti-infective regimen to facilitate resolution of the primary infection and associated symptoms and prevent suppurative complications or other sequelae, and state that judicious use of anti-infectives in the management of otitis media involves accurately diagnosing AOM and distinguishing AOM (which should be treated with anti-infectives) from otitis media with effusion (which usually is not treated with anti-infectives).
AOM usually is treated empirically with an anti-infective agent selected based on its efficacy against the most probable bacterial pathogens.
Other considerations in the choice of an anti-infective for empiric treatment of AOM include pharmacokinetic data related to distribution of the drug into middle ear fluid, compliance issues related to patient acceptance of dosage formulation and dosage schedule, adverse effects profiles, and cost considerations; drug susceptibility patterns in the local community can be considered, but local surveillance data are not necessarily representative of AOM isolates found in otherwise healthy patients.
Various anti-infectives, including oral amoxicillin, oral amoxicillin and clavulanate potassium, various oral cephalosporins (cefaclor, cefdinir, cefixime, cefpodoxime proxetil, cefprozil, ceftibuten, cefuroxime axetil, cephalexin), IM ceftriaxone, oral co-trimoxazole, oral erythromycin-sulfisoxazole, oral azithromycin, oral clarithromycin, and oral loracarbef, have been used in the treatment of AOM.
Traditionally, the drug of first choice for initial empiric treatment of AOM has been amoxicillin, unless the infection was suspected of being caused by β-lactamase-producing bacteria resistant to the drug, in which case amoxicillin and clavulanate potassium was recommended.
The fact that multidrug-resistant S. pneumoniae are being reported with increasing frequency should be considered when selecting an anti-infective agent for empiric treatment of AOM. The AAP, CDC, and other clinicians state that, despite the increasing prevalence of multidrug-resistant S. pneumoniae and presence of b-lactamase-producing H. influenzae or M. catarrhalis in many communities, amoxicillin remains the anti-infective of first choice for treatment of uncomplicated AOM since amoxicillin is highly effective, has a narrow spectrum of activity, is well distributed into middle ear fluid, and is well tolerated and inexpensive.
Amoxicillin (especially when given in dosages of 80-90 mg/kg daily) usually is effective in the treatment of AOM caused by S. pneumoniae, including infections involving strains with intermediate resistance to penicillins, and also usually is effective in the treatment of AOM caused by most strains of H. influenzae.
Because S. pneumoniae is the most frequent cause of AOM (25-50% of cases) and because AOM caused by S. pneumoniae is more likely to be severe and less likely to resolve spontaneously than AOM caused by H. influenzae or M. catarrhalis, it has been suggested that it may be more important to choose an empiric anti-infective based on its activity against S. pneumoniae rather than its activity against other possible pathogens.
Results of controlled clinical studies indicate that 10-day regimens of most oral anti-infectives used in the empiric treatment of AOM are equally effective, and there is no evidence that the overall response rate to anti-infectives with a broader spectrum of activity (e.g., second and third generation cephalosporins) is any better than that reported with amoxicillin or amoxicillin and clavulanate potassium.
Of the currently available oral cephalosporins, cefaclor, cefdinir, cefixime, cefpodoxime proxetil, cefprozil, ceftibuten, and cefuroxime axetil have been used effectively for the treatment of AOM in pediatric patients.
However, there is evidence that some cephalosporins (e.g., cefaclor, cefprozil) may be less effective than some other available agents for the treatment of AOM when b-lactamase-producing bacteria are present and some (e.g., cefixime, ceftibuten) may be less effective than some other available agents for the treatment of AOM when S. pneumoniae with reduced susceptibility to penicillin are present.
5-Day Oral Regimens for Acute Otitis Media
While anti-infectives traditionally have been administered for 7-10 days for the treatment of AOM, some clinicians suggest that a shorter duration of treatment (i.e., 5 days or less) can be effective and may increase compliance, decrease the risk of emergence of resistant bacteria, decrease the risk of adverse effects, and decrease costs.
There is evidence from controlled clinical studies in pediatric patients with AOM that the clinical response rate to 5-day regimens of certain oral cephalosporins (e.g., cefaclor, cefdinir, cefpodoxime proxetil, cefprozil, cefuroxime axetil) is similar to that of 10-day regimens of oral cephalosporins, amoxicillin, or amoxicillin and clavulanate potassium. The AAP and some clinicians suggest that these 5-day regimens can be considered for adults and children 2 years of age and older with mild, uncomplicated AOM, but further study is needed to more fully evaluate efficacy of short-term regimens in infants and young children since studies to date have included only a limited number of children younger than 2 years of age.
Some clinicians caution that short-term anti-infective regimens (i.e., 5 days or less) may not be appropriate for the treatment of AOM in children younger than 2 years of age or for patients with underlying disease, craniofacial abnormalities, recurrent or persistent AOM, or perforated tympanic membranes and spontaneous purulent drainage.
Single-dose IM Ceftriaxone for Acute Otitis Media
When ceftriaxone is used for the treatment of AOM, a single IM dose of the drug usually is given, although a 3-day regimen also has been used for the treatment of persistent or recurrent AOM.
The single-dose IM ceftriaxone regimen offers some practical advantages over 5- to 10-day oral anti-infective regimens since it provides a more convenient dosing schedule, ensures compliance, and can be administered to patients who have nausea and vomiting; however, IM ceftriaxone may be more costly than oral regimens and the drug has a spectrum of activity that is broader than necessary for the treatment of AOM. Some clinicians suggest that further study of the single-dose ceftriaxone regimen is needed to more fully assess the bacteriologic eradication rate, long-term efficacy, and rate of relapse, and to determine whether the single-dose regimen contributes to emergence of resistant organisms.
There is evidence from controlled studies in children 4-30 months of age with AOM that a single IM dose of ceftriaxone is as effective as a 10-day regimen of oral amoxicillin and clavulanate potassium; however, a lower clinical cure rate was reported with the single-dose ceftriaxone regimen in one study, and the manufacturer cautions that this should be considered when weighing the potential advantages of the regimen.
Persistent or Recurrent Acute Otitis Media
Optimal therapeutic regimens for patients with primary treatment failure or persistent AOM (an episode of AOM persisting beyond 3-6 days of therapy or recurring within a few days after discontinuance of anti-infective therapy) or for patients with recurrent AOM (3 or more episodes of AOM within a 6-month period or 4 or more episodes of AOM within a 12-month period) have not been identified.
Patients with AOM who fail to respond to an initial anti-infective regimen often also fail to respond to a subsequent regimen, regardless of the anti-infective used. While there is evidence that retreatment with the same anti-infective used in the prior regimen may be associated with a lower success rate than use of a different anti-infective, use of a higher dosage of amoxicillin and clavulanate potassium (80-90 mg/kg daily of amoxicillin) may be effective in patients who failed to respond to a lower dosage. In some cases of persistent or recurrent AOM, identification of the causative organism by tympanocentesis or culture of middle ear fluid drainage may be appropriate. Primary treatment failure of AOM occurs most frequently in children younger than 2 years of age.
While primary treatment failure and persistent AOM may be the result of infection with bacteria resistant to the anti-infective administered (e.g., penicillin-resistant S. pneumoniae, β-lactamase-producing H. influenzae), many cases appear to be related to other factors since results of tympanocentesis indicate that the causative organism(s) often are susceptible in vitro to the primary treatment regimen or, in some cases, no bacteria are isolated.
Risk factors for recurrent AOM include a family history of the infection, group day-care outside the home during the first 2 years of life, exposure to tobacco smoke associated with parental smoking, and use of pacifiers; there is some evidence that breastfeeding reduces the risk of recurrent AOM. In cases of documented treatment failure, the CDC and others recommend that the subsequent anti-infective be chosen based on its efficacy against b-lactamase-producing bacteria and its activity against multidrug-resistant S. pneumoniae. For the treatment of persistent or recurrent AOM in patients who fail to respond to a previous regimen, the CDC and other clinicians suggest that the drugs of choice are oral amoxicillin or oral amoxicillin and clavulanate potassium (80-90 mg/kg daily of amoxicillin), oral cefuroxime axetil, or IM ceftriaxone (1- or 3-day regimen).
Cefixime, cefpodoxime, co-trimoxazole, erythromycin-sulfisoxazole, clarithromycin, or azithromycin also has been used as second-line agents. Clindamycin is considered an alternative for the treatment of AOM caused by S. pneumoniae, but would be ineffective in infections caused by H. influenzae or M. catarrhalis.
Because S. pneumoniae resistant to amoxicillin also frequently are resistant to co-trimoxazole, clarithromycin, and azithromycin, these drugs may not be effective in patients with AOM who fail to respond to amoxicillin. In patients with severe or complicated AOM or recurrent treatment failure, consideration should be given to using myringotomy to obtain culture specimens to aid in selecting the most appropriate anti-infective. There is evidence that a 3-day regimen of IM ceftriaxone (50 mg/kg once daily) can be effective for the treatment of persistent or recurrent AOM in pediatric patients 3 months of age or older with infections that failed to respond to treatment with other anti-infectives (e.g., amoxicillin, amoxicillin and clavulanate potassium, cefaclor, cefuroxime axetil).
The 3-day ceftriaxone regimen has been effective for the treatment of persistent or relapsing otitis media caused by H. influenzae, M. catarrhalis, S. pyogenes, or penicillin-susceptible S. pneumoniae; however, a few treatment failures have been reported when the causative agent was S. pneumoniae with reduced susceptibility to penicillin.
Prevention of Recurrent Acute Otitis Media
Anti-infectives (e.g., amoxicillin, sulfisoxazole) have been administered as long-term prophylaxis or suppressive therapy in an attempt to prevent recurrence of AOM or have been administered intermittently as prophylaxis at the first sign of an upper respiratory tract infection in children with a history of recurrent AOM. Although it has been suggested and there is some evidence that anti-infective prophylaxis may decrease the incidence of new symptomatic episodes of AOM in some children with a history of recurrent AOM, such prophylaxis is not routinely recommended for children with recurrent AOM.
Results of a meta-analysis indicate that use of anti-infective prophylaxis results in an average decrease of only 0.11 episodes of AOM per patient per month (slightly more than 1 episode per year). In addition, there are concerns that anti-infective prophylaxis in patients with recurrent AOM promotes emergence of resistant bacteria, including multidrug-resistant S. pneumoniae, and such prophylaxis may alter the nasopharyngeal flora and foster colonization with resistant bacteria which would compromise therapeutic efficacy of the prophylactic drug.
Although no longer routinely recommended, some clinicians suggest that use of anti-infective prophylaxis may be considered for selected children with 3 or more documented episodes of AOM within a 6-month period or 4 or more episodes within a 12-month period and also can be considered for children who have an episode of AOM within the first 6 months of life or 2 episodes within the first year of life if they have a family history of ear infections.
If anti-infective prophylaxis is used in selected patients with recurrent AOM, some clinicians suggest that the most effective regimen involves continuous administration for no longer than 6 months during the fall, winter, or early spring months when respiratory tract infections are most frequent; either sulfisoxazole or amoxicillin is recommended, but nasopharyngeal colonization with resistant S. pneumoniae appears to occur more frequently in those receiving amoxicillin. In a retrospective study evaluating use of prophylactic anti-infectives in pediatric patients 1 month to 15 years of age with a history of recurrent AOM, patients received a 10-day regimen of oral amoxicillin or oral cefaclor for treatment of the acute episode and then a suppressive regimen of amoxicillin (20 mg/kg once daily) or cefaclor (20 mg/kg once daily) for a mean duration of 8.6 months (range: 3-20 months).
Results indicate that suppressive therapy failed in 47% of those receiving cefaclor and 70% of those receiving amoxicillin; most of these patients required other interventions (e.g., placement of tympanostomy tubes). In addition, in a placebo-controlled study in children 3 months to 6 years of age with recurrent AOM, amoxicillin prophylaxis (20 mg/kg daily given in 1 or 2 divided doses) did not result in a lower incidence of new episodes of AOM.
Otitis Media with Effusion
Some cephalosporins (e.g., cefaclor, ceftibuten) have been used in the treatment of otitis media with effusion (OME); however, anti-infectives are not usually recommended for the initial treatment of OME. OME (also referred to as noninfected or nonsuppurative otitis media, secretory otitis media, serous otitis media, middle ear effusion, fluid ear, glue ear) is defined as the presence of residual or persistent middle ear effusion without signs or symptoms of infection.
OME may occur following an episode of AOM or as a result of eustachian tube obstruction following a viral upper respiratory tract infection. The pathogenesis of OME is multifactorial, and the role of bacteria in OME is not completely understood.
While some studies report that cultures of middle ear effusion from patients with OME rarely indicate the presence of bacteria, results of other studies using other methods (e.g., polymerase chain reaction testing) suggest the presence of bacteria, including Alloiococcus otitis (a recently recognized gram-positive cocci), in effusion fluid of patients with OME. In most patients with acute AOM who receive appropriate treatment with anti-infectives, middle ear effusions usually are sterilized within 2-6 days but the effusions may persist for weeks or months before eventually resolving spontaneously without further treatment
. In children who have received appropriate treatment for an episode of AOM, approximately 70% will have middle ear effusion at 2 weeks, 50% have effusion at 1 month, and 20% have effusion at 2 months. In a group of children 2-6 years of age in group child-care who had OME, 80% had clearance of effusion within 2 months. Chronic OME (middle ear effusion present continuously for over 3 months) may occur in some children and can be associated with conductive hearing loss, which may adversely affect language development and academic performance.
Risk factors for chronic OME include attendance in group day-care outside the home, age younger than 2 years of age, and exposure to tobacco smoke associated with parental smoking. Optimum strategies for the management of OME that persists for 3 months or longer have not been identified and use of available strategies (e.g., anti-infective therapy, surgical intervention) remain controversial.
There is some evidence that surgical intervention (e.g., adenoidectomy, adenotonsillectomy, insertion of tympanostomy tubes) may shorten the time to resolution of severe, chronic OME in children and may provide some benefits in terms of language development, but anti-infectives appear to provide only a limited benefit in terms of enhancing resolution of effusion.
Because there is little evidence that anti-infective therapy provides any long-term benefits and because of concerns that use of anti-infectives in patients with OME may promote emergence of resistant bacteria, some clinicians suggest that management of OME not include the use of anti-infectives; however, others suggest that anti-infectives be used in selected patients. Guidelines for the diagnosis and treatment of OME have been developed by The US Agency for Healthcare Research and Quality (AHRQ, formerly the US Agency for Health Care Policy and Research, AHCPR) in consultation with the AAP, the American Academy of Family Physicians (AAFP), and the American Academy of Otolaryngology—Head and Neck Surgery.
These guidelines include an algorithm for managing OME in an otherwise healthy child 1-3 years of age and should be consulted for additional information regarding diagnosis and management, including the role of anti-infectives and surgical intervention. While the AHRQ guidelines suggest that use of anti-infectives can be considered in the management of OME when the effusion has persisted for at least 6 weeks, many clinicians recommend that anti-infectives be administered only when OME occurs in association with acute ear pain, the serous effusion changes to a purulent effusion, or the effusion persists for more than 3 months and is associated with conductive hearing loss (hearing threshold of 20 decibels or worse).
The AAP states that anti-infectives may be indicated if the effusion persists for 3 months or longer. If an anti-infective is used in patients with OME, some clinicians recommend use of amoxicillin or amoxicillin and clavulanate potassium. In a study in children 7 months to 12 years of age with OME, a 14-day regimen of oral ceftibuten or oral amoxicillin resulted in resolution of middle ear effusion (based on otoscopy) in 27-30% of children, but there was recurrence of effusion at 16-week follow-up in 60-67% of children who were effusion free at completion of therapy. In a placebo-controlled study evaluating 14-day regimens of amoxicillin, cefaclor, or erythromycin-sulfisoxazole in children with OME, middle ear effusion had resolved by the end of the treatment period in 22% of those who received cefaclor, 21% of those who received erythromycin-sulfisoxazole, and 31.% of those who received amoxicillin; of those who were effusion-free at 4 weeks, there was recurrence of effusion during the next 12 weeks in 52% of those who received cefaclor, 47% of those who received erythromycin-sulfisoxazole, and 60.% of those who received amoxicillin.
Chronic Suppurative Otitis Media without Cholesteatoma
Ceftazidime has been used with some success in the treatment of chronic suppurative otitis media (CSOM) without cholesteatoma. CSOM is defined as chronic infection of the middle ear and mastoid associated with tympanic membrane perforation and otorrhea lasting more than 6 weeks, and may occur as the result of unresolved AOM and/or eustachian tube dysfunction.
The most common bacteria reported in patients with CSOM are Ps. aeruginosa, Klebsiella, S. aureus, S. epidermidis, and anaerobic bacteria, including Bacteroides, Prevotella, Peptostreptococcus, and Peptococcus.
While ceftazidime has been effective in the treatment of CSOM when gram-negative bacteria were involved, it has been ineffective when gram-positive bacteria (e.g., S. aureus) were involved. Because CSOM often is a mixed aerobic-anaerobic infection, anti-infectives usually used in the treatment of AOM or OME would be ineffective. Topical anti-infectives (e.g., ciprofloxacin otic suspension, ofloxacin otic solution, gentamicin) used in conjunction with daily aural toilet can be effective for the treatment of uncomplicated CSOM; more severe or persistent infections require treatment with a parenteral anti-infective (e.g., ceftazidime, clindamycin, ciprofloxacin, gentamicin, ticarcillin, ticarcillin disodium and clavulanate potassium).
Otitis Externa
Ceftazidime has been effective when used in the treatment of malignant otitis externa caused by Ps. aeruginosa. Bacterial otitis externa usually is caused by Ps. aeruginosa or S. aureus. Although acute bacterial otitis externa localized in the external auditory canal may be effectively treated using topical anti-infectives (e.g., ciprofloxacin otic suspension, ofloxacin otic solution), malignant otitis externa is an invasive, potentially life-threatening infection, especially in immunocompromised patients such as those with diabetes mellitus or human immunodeficiency virus (HIV) infection, and requires prompt diagnosis and long-term treatment with parenteral anti-infectives (e.g., ceftazidime and/or ciprofloxacin).
Pharyngitis and Tonsillitis
Oral cephalosporins (e.g., cefaclor, cefadroxil, cefdinir, cefditoren pivoxil, cefixime, cefpodoxime proxetil, cefprozil, ceftibuten, cefuroxime axetil, cephalexin, cephradine) are used for the treatment of pharyngitis and tonsillitis caused by S. pyogenes (group A b-hemolytic streptococci).
Although cephalosporins usually are effective in eradicating S. pyogenes from the nasopharynx, efficacy of the drugs in the subsequent prevention of rheumatic fever remains to be established. Selection of an anti-infective agent regimen for the treatment of S. pyogenes pharyngitis and tonsillitis should be based on the drug’s spectrum of activity as well as the regimen’s bacteriologic and clinical efficacy, potential adverse effects, ease of administration and patient compliance, and cost. No regimen has been found to date that effectively eradicates group A b-hemolytic streptococci in 100% of patients.
Because penicillin has a narrow spectrum of activity, is inexpensive, and generally is effective, the CDC, AAP, AAFP, Infectious Diseases Society of American (IDSA), 580 American Heart Association (AHA), American College of Physicians-American Society of Internal Medicine (ACP-ASIM), and others consider natural penicillins (i.e., 10 days of oral penicillin V or a single IM dose of penicillin G benzathine) the treatment of choice for streptococcal pharyngitis and tonsillitis and prevention of initial attacks (primary prevention) of rheumatic fever, although oral amoxicillin often is used instead of penicillin V in small children because of a more acceptable taste. Other anti-infectives (e.g., oral cephalosporins, oral macrolides) generally are considered to be alternative agents.
There is some evidence that bacteriologic and clinical cure rates reported with 10-day regimens of certain oral cephalosporins (e.g., cefaclor, cefadroxil, cefdinir, cefixime, cefpodoxime proxetil, cefprozil, cefuroxime axetil, ceftibuten, cephalexin) are slightly higher than those reported with the 10-day oral penicillin V regimen. In addition, there is some evidence that a shorter duration of therapy with certain oral cephalosporins (e.g., a 5-day regimen of cefadroxil, cefdinir, cefixime, or cefpodoxime proxetil or a 4- or 5-day regimen of cefuroxime axetil) achieves bacteriologic and clinical cure rates equal to or greater than those achieved with the traditional 10-day oral penicillin V regimen.
Based on these results, some clinicians have suggested that oral cephalosporins should be included as agents of choice for the treatment of S. pyogenes pharyngitis and tonsillitis. However, there is some controversy concerning study design (e.g., clinical status of patients prior to treatment, definition of treatment failure, compliance issues, timing of follow-up cultures) of some of the earlier studies that evaluated efficacy of penicillin regimens.
The IDSA states that first generation cephalosporins can be used for the treatment of pharyngitis in patients hypersensitive to penicillins (except in those with immediate-type hypersensitivity to ?-lactam anti-infectives) but that cephalosporins appear to offer no advantage over penicillins since they have a broader spectrum of activity and generally are more expensive. In addition, because of limited data to date, the IDSA states that use of cephalosporin regimens administered for 5 days or less for the treatment of S. pyogenes pharyngitis cannot be recommended at this time.
For additional information on treatment of S. pyogenes pharyngitis, see Pharyngitis and Tonsillitis under Gram-positive Aerobic Bacterial Infections: Streptococcus pyogenes Infections, in Uses in the Natural Penicillins General Statement 8:12..04.
Endocarditis
IM or IV ceftriaxone is used for the treatment of native valve endocarditis caused by penicillin-susceptible viridans streptococci (e.g., S. milleri, S. mitis, S. mutans, S. salivarius, S. sanguis) or nonenterococcal group D streptococcus (S. bovis). Parenteral ceftriaxone also is used for the treatment of native valve or prosthetic valve endocarditis caused by slow-growing fastidious gram-negative bacilli termed the HACEK group (i.e., Haemophilus parainfluenzae, H. aphrophilus, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella kingae). (See Uses: Endocarditis in Ceftriaxone 8:12.06.12.)
Oral cefadroxil, IM or IV cefazolin, or oral cephalexin is used as an alternative to amoxicillin or ampicillin for prevention of a-hemolytic (viridans group) streptococcal endocarditis in penicillin-allergic individuals considered at risk for bacterial endocarditis following certain dental or upper respiratory tract procedures. For information regarding indications for prophylaxis against bacterial endocarditis, see Uses: Prevention of Bacterial Endocarditis, in the Aminopenicillins General Statement 8:12.16.08.
GI Infections
Some parenteral third generation cephalosporins are used in the treatment of GI infections caused by Salmonella or Shigella or the treatment of other uncommon infectious diarrheal illnesses, including infections caused by Vibrio and Yersinia.
Salmonella Gastroenteritis
Anti-infective therapy generally is not indicated in otherwise healthy individuals with uncomplicated (noninvasive) gastroenteritis caused by non-typhi Salmonella (e.g., S. enteritidis, S. typhimurium) since these infections generally subside spontaneously and there is some evidence that such therapy may prolong the duration of fecal excretion of the organisms; however, the CDC, AAP, IDSA, and others recommend anti-infective therapy in individuals with severe Salmonella gastroenteritis and in those who are at increased risk of invasive disease.
These individuals at increased risk include infants younger than 3-6 months of age; individuals older than 50 years of age; individuals with hemoglobinopathies, severe atherosclerosis or valvular heart disease, prostheses, uremia, chronic GI disease, or severe colitis; and individuals who are immunocompromised because of malignancy, immunosuppressive therapy, HIV infection, or other immunosuppressive illness.
However, no controlled studies have demonstrated a beneficial effect of such treatment and there is some evidence that anti-infective therapy can prolong the duration of fecal excretion of the organism. When an anti-infective agent is considered necessary in an individual with Salmonella gastroenteritis, the CDC, AAP, IDSA, and others recommend use of ceftriaxone, cefotaxime, a fluoroquinolone (should be used in children only if the benefits outweigh the risks and no other alternative exists), ampicillin, amoxicillin, co-trimoxazole, or chloramphenicol, depending on the susceptibility of the causative organism. (See Uses: Typhoid Fever and Other Salmonella Infections in Ceftriaxone 8:12.06.12.)
Shigella Infections
Ceftriaxone is used for the treatment of shigellosis caused by susceptible Shigella sonnei or S. flexneri and is an alternative to fluoroquinolones for treatment of these infections in pediatric patients or when susceptibility is unknown or strains resistant to ampicillin and co-trimoxazole are isolated. (See Uses: Shigella Infections, in Ceftriaxone 8:12.06.12.)
Vibrio Infections
Cefotaxime is one of several alternatives recommended for the treatment of severe cases of Vibrio parahaemolyticus infection when anti-infective therapy is indicated in addition to supportive care. V. parahaemolyticus infections can occur as the result of ingestion of contaminated undercooked or raw fish or shellfish, and some clinicians recommend use of tetracycline, doxycycline, gentamicin, or cefotaxime when treatment is considered necessary. (See Uses Vibrio Infections, in Cefotaxime 8:12.06.12.)
Cefotaxime and ceftazidime are considered drugs of choice for the treatment of infections caused by V. vulnificus; these infections can occur as the result of ingesting raw or undercooked seafood (especially raw oysters) or through contamination of a wound with seawater or seafood drippings. While optimum anti-infective therapy for the treatment of V. vulnificus infections has not been identified, use of cefotaxime, ceftazidime, tetracycline, or doxycycline is recommended.
Yersinia Infections
Cefotaxime and ceftizoxime are suggested as possible choices for the treatment of GI infections caused by Yersinia enterocolitica or Y. pseudotuberculosis.
These Yersinia infections usually are self-limited and anti-infective therapy unnecessary; however, the AAP, IDSA, and others recommend use of anti-infectives in immunocompromised individuals or for the treatment of severe infections or when septicemia or other invasive disease occurs. GI infections caused by Y. enterocolitica or Y. pseudotuberculosis can occur as the result of ingesting undercooked pork, unpasteurized milk, or contaminated water; infection has occurred in infants whose caregivers handled contaminated chitterlings (raw pork intestines) or tofu.
Use of co-trimoxazole, an aminoglycoside (amikacin, gentamicin, tobramycin), a fluoroquinolone (e.g., ciprofloxacin), doxycycline, cefotaxime, or ceftizoxime has been recommended when treatment is considered necessary; combination therapy may be necessary. Some clinicians suggest that the role of oral anti-infectives in the management of enterocolitis, pseudoappendicitis syndrome, or mesenteric adenitis caused by Yersinia needs further evaluation.
Intra-abdominal Infections
Cefoperazone, cefotaxime, ceftazidime, ceftizoxime, and ceftriaxone are used parenterally for the treatment of intra-abdominal infections and for the treatment of various obstetric and gynecologic infections, including pelvic inflammatory disease. (See Uses: Pelvic Inflammatory Disease.) While ceftazidime or ceftriaxone monotherapy may be effective in some patients for the treatment of mixed aerobic-anaerobic intra-abdominal infections, the drugs generally should not be used alone for the treatment of serious intra-abdominal infections when B. fragilis may be present. IV cefepime is used in conjunction with IV metronidazole for the treatment of complicated intra-abdominal infections caused by E. coli, viridans streptococci,Ps. aeruginosa, K. pneumoniae, Enterobacter, or B. fragilis.
Meningitis and Other CNS Infections
IV cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, and cefuroxime are used in adult or pediatric patients for the treatment of meningitis caused by susceptible H. influenzae, Neisseria meningitidis, or S. pneumoniae; however, cefotaxime or ceftriaxone generally are preferred when a cephalosporin is indicated for the treatment of meningitis caused by these organism. IV cefotaxime, ceftizoxime, and ceftriaxone are used alone or in conjunction with an aminoglycoside for the treatment of meningitis or other CNS infections caused by susceptible Enterobacteriaceae (e.g., E. coli, Klebsiella) and IV ceftazidime is used in conjunction with an aminoglycoside for the treatment of meningitis caused by susceptible Ps. aeruginosa.
Empiric Treatment of Meningitis
Pending results of CSF culture and in vitro susceptibility testing, the most appropriate anti-infective regimen for empiric treatment of suspected bacterial meningitis should be selected based on results of CSF Gram stain and antigen tests, age of the patient, the most likely pathogen(s) and source of infection, and current patterns of bacterial resistance within the hospital and local community.
When results of culture and susceptibility tests become available and the pathogen is identified, the empiric anti-infective regimen should be modified (if necessary) to ensure that the most effective regimen is being administered. There is some evidence that short-term adjunctive therapy with IV dexamethasone may decrease the incidence of audiologic and/or neurologic sequelae in infants and children with H. influenzae meningitis and possibly may provide some benefit in patients with S. pneumoniae meningitis.
While such therapy is controversial, the AAP and other clinicians suggest that use of adjunctive dexamethasone therapy should be considered during the initial 2-4 days of anti-infective therapy in infants and children 6-8 weeks of age or older with known or suspected bacterial meningitis and is recommended in those with suspected or proven H. influenzae infection. (SeeUses: Bacterial Meningitis in the Corticosteroids General Statement 68:04 and see Dexamethasone 68:04.) Bacterial meningitis in neonates usually is caused by S. agalactiae (group B streptococci), Listeria monocytogenes, or aerobic gram-negative bacilli (e.g., E. coli, K. pneumoniae). The AAP recommends that neonates with suspected bacterial meningitis receive an empiric regimen of IV ampicillin and an aminoglycoside pending results of CSF culture and susceptibility testing. Alternatively, neonates can receive an empiric regimen of IV ampicillin and IV cefotaxime or IV ceftazidime with or without gentamicin.
Because frequent use of cephalosporins in neonatal units may result in rapid emergence of resistant strains of some gram-negative bacilli (e.g., Enterobacter cloacae, Klebsiella, Serratia), the AAP cautions that cephalosporins should be used for empiric treatment of meningitis in neonates only if gram-negative bacterial meningitis is strongly suspected. Consideration should be given to including IV vancomycin in the empiric regimen if S. pneumoniae, enterococci, or Staphylococci is suspected.
Because ceftriaxone should be used with caution in neonates who are hyperbilirubinemic (especially those born prematurely), cefotaxime may be the preferred cephalosporin for empiric treatment of meningitis is neonates.
However, because premature, low-birthweight neonates are at increased risk for nosocomial infection caused by staphylococci or gram-negative bacilli, some clinicians suggest that these neonates receive an empiric regimen of IV ceftazidime and IV vancomycin. In infants beyond the neonatal stage who are younger than 3 months of age, bacterial meningitis usually is caused by S. agalactiae, L. monocytogenes, H. influenzae, S. pneumoniae, N. meningitidis, or aerobic gram-negative bacilli (e.g., E. coli, K. pneumoniae). The empiric regimen recommended for infants in this age group is IV ampicillin and either IV ceftriaxone or IV cefotaxime. Consideration should be given to including IV vancomycin in the empiric regimen if S. pneumoniae is suspected. In children 3 months through 17 years of age, bacterial meningitis usually is caused by N. meningitidis, S. pneumoniae, or H. influenzae, and the most common cause of bacterial meningitis in adults 18-50 years of age is N. meningitidis or S. pneumoniae.
An empiric regimen of IV ceftriaxone or IV cefotaxime usually is used for empiric therapy of suspected bacterial meningitis in children 3 months through 17 years of age and in adults 18-50 years of age. Although an empiric regimen of IV ampicillin and IV chloramphenicol can be used as an alternative regimen in children 3 months through 17 years of age, most clinicians prefer a cephalosporin regimen unless the drugs are contraindicated.
Because of the increasing prevalence of penicillin-resistant S. pneumoniae that also are resistant to or have reduced susceptibility to cephalosporins, the AAP and others recommend that the initial empiric cephalosporin regimen include IV vancomycin (with or without rifampin) pending results of in vitro susceptibility tests; vancomycin and rifampin should be discontinued if the causative organism is found to be susceptible to the cephalosporin.
The CDC and some clinicians have recommended that vancomycin be added to the empiric regimen in areas where there have been reports of highly penicillin-resistant strains of S. pneumoniae, but other clinicians suggest that use of ceftriaxone or cefotaxime in conjunction with vancomycin provides the optimal initial empiric regimen. While L. monocytogenes meningitis is relatively rare in this age group, the empiric regimen should include ampicillin if L. monocytogenes is suspected. In adults older than 50 years of age, bacterial meningitis usually is caused by S. pneumoniae, L. monocytogenes, N. meningitidis, or aerobic gram-negative bacilli, and the empiric regimen recommended for this age group is IV ampicillin given in conjunction with IV cefotaxime or IV ceftriaxone.
Because of the increasing prevalence of penicillin-resistant S. pneumoniae, some clinicians suggest that the empiric regimen also should include IV vancomycin (with or without rifampin).
Meningitis Caused by Streptococcus pneumoniae
IV ceftriaxone and IV cefotaxime are considered drugs of choice for the treatment of meningitis caused by susceptible S. pneumoniae. While cefotaxime and ceftriaxone generally have been considered the drugs of choice for the treatment of meningitis caused by penicillin-resistant S. pneumoniae, treatment failures have been reported when the drugs were used alone for the treatment of meningitis caused by strains of S. pneumoniae with intermediate or high-level penicillin resistance (i.e., penicillin MIC 0.1 mcg/mL or greater).
In addition, strains of S. pneumoniae with reduced susceptibility to cephalosporins have been reported with increasing frequency, and use of cefotaxime or ceftriaxone alone may be ineffective for the treatment of meningitis caused by these strains. The prevalence of S. pneumoniae with reduced susceptibility to penicillin and/or cephalosporins varies geographically, and clinicians should be aware of the prevalence and pattern of S. pneumoniae drug resistance in the local community to optimize empiric regimens and initial therapy for serious pneumococcal infections.
Because susceptibility can no longer be assumed, S. pneumoniae isolates should be routinely tested for in vitro susceptibility. If anti-infective therapy in a patient with meningitis is initiated with an empiric regimen of IV ceftriaxone or IV cefotaxime and IV vancomycin (with or without rifampin) and results of culture and in vitro susceptibility testing indicate that pathogen involved is a strain of S. pneumoniae susceptible to the cephalosporin and susceptible or resistant to penicillin, vancomycin and rifampin can be discontinued and therapy completed using ceftriaxone or cefotaxime alone.
If the isolate is found to have reduced susceptibility to ceftriaxone and cefotaxime and penicillin, the IV cephalosporin and IV vancomycin usually are both continued. If the patient’s condition does not improve or worsens or results of a second repeat lumbar puncture (performed 24-48 hours after initiation of anti-infective therapy) indicate that the anti-infective regimen has not eradicated or substantially reduced the number of pneumococci in CSF, rifampin probably should be added to the regimen or vancomycin discontinued and replaced with rifampin. If meningitis is caused by S. pneumoniae highly resistant to ceftriaxone (i.e., MIC 4 mcg/mL or greater), consultation with an infectious disease expert is recommended.
Meningitis Caused by Haemophilus influenzae
IV ceftriaxone and IV cefotaxime are considered drugs of choice for the initial treatment of meningitis caused by susceptible H. influenzae (including penicillinase-producing strains). The AAP suggests that children with meningitis possibly caused by H. influenzae can receive an initial treatment regimen of ceftriaxone, cefotaxime, or a regimen of ampicillin given in conjunction with chloramphenicol; some clinicians prefer ceftriaxone or cefotaxime for the initial treatment of meningitis caused by H. influenzae since these cephalosporins are active against both penicillinase-producing and nonpenicillinase-producing strains.
Because of the prevalence of ampicillin-resistant H. influenzae, ampicillin should not be used alone for empiric treatment of meningitis when H. influenzae may be involved. The incidence of H. influenzae meningitis in the US has decreased considerably since H. influenzae type b conjugate vaccines became available for immunization of infants.
Meningitis Caused by Neisseria meningitidis
While both IV ampicillin and IV penicillin G may be used for the treatment of meningitis caused by N. meningitidis, the AAP and other clinicians suggest that IV penicillin G is the drug of choice for the treatment of these infections and IV ceftriaxone and IV cefotaxime are acceptable alternatives. Chloramphenicol is recommended for the treatment of N. meningitidis meningitis in patients with a history of anaphylactoid-type hypersensitivity reactions to penicillin.
Meningitis Caused by Enterobacteriaceae
Some clinicians recommend that meningitis caused by Enterobacteriaceae (e.g., E. coli, K. pneumoniae) be treated with a third generation cephalosporins (i.e., cefotaxime, ceftazidime, ceftriaxone) with or without an aminoglycoside. Because ceftazidime (but not cefotaxime or ceftriaxone) is effective for the treatment of meningitis caused by Ps. aeruginosa, some clinicians suggest that a regimen of ceftazidime and an aminoglycoside may be preferred for the treatment of meningitis caused by gram-negative bacilli pending results of culture and susceptibility testing.
Meningitis Caused by Pseudomonas aeruginosa
In patients with meningitis caused by Ps. aeruginosa, most clinicians recommend that therapy be initiated with a regimen of ceftazidime and a parenteral aminoglycoside. If the patient fails to respond to this regimen, concomitant use of intrathecal or intraventricular aminoglycoside therapy or use of an alternative parenteral anti-infective (e.g., aztreonam, meropenem, a quinolone) should be considered based on results of in vitro susceptibility tests. When treating pediatric patients with meningitis caused by Ps. aeruginosa or Enterobacteriaceae, consultation with an infectious disease expert may be beneficial.
Meningitis Caused by Streptococcus agalactiae
For the initial treatment of meningitis or other severe infection caused by S. agalactiae (group B streptococci), a regimen of IV ampicillin or IV penicillin G given in conjunction with an aminoglycoside is recommended. Some clinicians suggest that IV ampicillin is the drug of choice for the treatment of group B streptococcal meningitis and that an aminoglycoside (IV gentamicin) should be used concomitantly during the first 72 hours until in vitro susceptibility testing is completed and a clinical response if observed; thereafter, ampicillin can be given alone.
Meningitis Caused by Listeria monocytogenes
The optimal regimen for the treatment of meningitis caused by L. monocytogenes has not been established. Cephalosporins are not active against Listeria monocytogenes, an organism that most frequently causes meningitis in neonates or immunocompromised individuals, and the drugs should not be used alone for empiric treatment of meningitis when this organisms may be involved. The AAP and other clinicians generally recommend that meningitis or other severe infection caused by L. monocytogenes be treated with a regimen of IV ampicillin with or without an aminoglycoside (usually gentamicin); alternatively, a regimen of penicillin G used in conjunction with gentamicin can be used. In patients hypersensitive to penicillin, the alternative regimen for treatment of meningitis caused by L. monocytogenes is co-trimoxazole.
Brain Abscess and Other CNS Infections
Bacterial brain abscesses and other CNS infections (e.g., subdural empyema, intracranial epidural abscesses) often are polymicrobial and can be caused by gram-positive aerobic cocci, Enterobacteriaceae (e.g., E. coli, Klebsiella), and/or anaerobic bacteria (e.g., Bacteroides, Fusobacterium).
The choice of anti-infectives for empiric therapy of these infections should be based on the predisposing condition and site of primary infection.
Some clinicians suggest that the empiric anti-infective regimen in patients who develop the CNS infections after respiratory tract infection (e.g., otitis media, mastoiditis, paranasal sinusitis, pyogenic lung disease) should consist of an appropriate third generation IV cephalosporin (e.g., ceftriaxone, cefotaxime, ceftazidime) given in conjunction with metronidazole; employing one of these cephalosporins rather than a penicillin provides coverage against Haemophilus and facultative anaerobic gram-negative bacteria.
If presence of staphylococci is suspected, a penicillinase-resistant penicillin (e.g., nafcillin, oxacillin) or vancomycin should be added to the empiric regimen. In patients who develop brain abscess, subdural empyema, or intracranial epidural abscess after trauma or neurosurgery, the empiric regimen should consist of an appropriate third generation IV cephalosporin (e.g., ceftriaxone, cefotaxime, ceftazidime) given in conjunction with a penicillinase-resistant penicillin or vancomycin. Prolonged anti-infective therapy (e.g., 3-6 weeks or longer) usually is required for the treatment of brain abscess, subdural empyema, or intracranial epidural abscess.
Respiratory Tract Infections
Community-acquired Pneumonia
Some oral cephalosporins (cefdinir, cefpodoxime proxetil, cefprozil, cefuroxime axetil) and some parenteral cephalosporins (cefepime, cefotaxime, ceftriaxone) are used in the treatment of community-acquired pneumonia (CAP). Initial treatment of CAP generally involves use of an empiric anti-infective regimen based on the most likely pathogens; therapy may then be changed (if possible) to a pathogen-specific regimen based on results of in vitro culture and susceptibility testing, especially in hospitalized patients.
The most appropriate empiric regimen varies depending on the severity of illness at the time of presentation and whether outpatient treatment or hospitalization in or out of an intensive care unit (ICU) is indicated and the presence or absence of cardiopulmonary disease and other modifying factors that increase the risk of certain pathogens (e.g., penicillin- or multidrug-resistant S. pneumoniae, enteric gram-negative bacilli, Ps. aeruginosa).
For both outpatients and inpatients, most experts recommend that an empiric regimen for the treatment of CAP includes an anti-infective active against S. pneumoniae since this organism is the most commonly identified cause of bacterial pneumonia and causes more severe disease than many other common CAP pathogens.
Outpatient Regimens for CAP
Pathogens most frequently involved in outpatient CAP include S. pneumoniae, Mycoplasma pneumoniae, Chlamydia pneumoniae, respiratory viruses, and Haemophilus influenzae (especially in cigarette smokers).
Therefore, for empiric outpatient treatment of acute CAP in immunocompetent adults, the IDSA recommends monotherapy with an oral macrolide (azithromycin, clarithromycin, erythromycin), oral doxycycline, or an oral fluoroquinolone active against S. pneumoniae (e.g., gatifloxacin, levofloxacin, moxifloxacin) and states that alternative empiric regimens include oral amoxicillin and clavulanate or certain oral cephalosporins (cefpodoxime, cefprozil, cefuroxime axetil).
The IDSA states that, when an oral cephalosporin is used for the treatment of CAP, cefpodoxime, cefprozil, or cefuroxime axetil generally is preferred over other possibilities because of their in vitro activity against S. pneumoniae. For outpatient treatment of CAP in immunocompetent adults without cardiopulmonary disease or other modifying factors that would increase the risk of multidrug-resistant S. pneumoniae or gram-negative bacteria, the American Thoracic Society (ATS) recommends an empiric regimen of monotherapy with azithromycin or clarithromycin or, alternatively, doxycycline.
However, for the outpatient treatment of immunocompetent adults with cardiopulmonary disease (congestive heart failure or chronic obstructive pulmonary disease [COPD]) and/or other modifying factors that increase the risk for multidrug-resistant S. pneumoniae or gram-negative bacteria, the ATS recommends a 2-drug empiric regimen consisting of a β-lactam anti-infective (e.g. oral cefpodoxime, oral cefuroxime axetil, high-dose amoxicillin, amoxicillin and clavulanate, parenteral ceftriaxone followed by oral cefpodoxime) and a macrolide or doxycycline or, alternatively, monotherapy with a fluoroquinolone active against S. pneumoniae (e.g., ciprofloxacin, ofloxacin, gatifloxacin, levofloxacin, moxifloxacin, sparfloxacin, trovafloxacin [risk of hepatic toxicity should be considered]).
The CDC suggests that use of these oral fluoroquinolones in the outpatient treatment of CAP be reserved for when other anti-infectives are ineffective or cannot be used or when highly penicillin-resistant S. pneumoniae (i.e., penicillin MICs 4 mcg/mL or greater) are identified as the cause of infection.
Inpatient Regimens for CAP
In addition to S. pneumoniae, other pathogens often involved in inpatient CAP are H. influenzae, enteric gram-negative bacilli, S. aureus, Legionella, M. pneumoniae, C. pneumoniae, and viruses.
Patients with severe CAP admitted into the ICU may have Ps. aeruginosa infections (especially those with underlying bronchiectasis or cystic fibrosis) and Enterobacteriaceae often are involved.
In addition, anaerobic infection should be suspected in patients with aspiration pneumonia or lung abscess. Inpatient treatment of CAP is initiated with a parenteral regimen, although therapy may be changed to an oral regimen if the patient is improving clinically, is hemodynamically stable, and able to ingest drugs. CAP patients usually have a clinical response within 3-5 days after initiation of therapy and failure to respond to the initial empiric regimen generally indicates an incorrect diagnosis, host failure, inappropriate anti-infective regimen (drug selection, dosage, route), unusual pathogen, adverse drug reaction, or complication (e.g., pulmonary superinfection, empyema).
For empiric inpatient treatment of CAP in immunocompetent adults who require hospitalization in a general patient-care setting (not an ICU), the IDSA recommends a 2-drug regimen consisting of a parenteral β-lactam anti-infective (e.g., cefotaxime, ceftriaxone, ampicillin and sulbactam, piperacillin and tazobactam) and a macrolide (e.g., azithromycin, clarithromycin, erythromycin) or monotherapy with a fluoroquinolone active against S. pneumoniae (e.g., gatifloxacin, levofloxacin, moxifloxacin).
For empiric inpatient treatment of CAP in immunocompetent adults who are hospitalized in a general patient-care setting and have cardiopulmonary disease (congestive heart failure or chronic obstructive pulmonary disease [COPD]) and/or other modifying factors that increase the risk for multidrug-resistant S. pneumoniae or gram-negative bacteria, the ATS recommends a 2-drug regimen consisting of a parenteral b-lactam anti-infective (cefotaxime, ceftriaxone, ampicillin and sulbactam, high-dose ampicillin) and an oral or IV macrolide (azithromycin or clarithromycin; doxycycline can be used in those with macrolide sensitivity or intolerance) or, alternatively, monotherapy with an IV fluoroquinolone active against S. pneumoniae.
If anaerobes are documented or lung abscess is present, clindamycin or metronidazole should be added to the regimen. For CAP patients admitted to a general patient-care setting who do not have cardiopulmonary disease or other modifying factors, the ATS suggests an empiric regimen of monotherapy with IV azithromycin; for those with macrolide sensitivity or intolerance, a 2-drug regimen of doxycycline and a b-lactam or monotherapy with a fluoroquinolone active against S. pneumoniae can be used.
For inpatient treatment of CAP in immunocompetent adults who require hospitalization in an ICU, the IDSA recommends an empiric 2-drug regimen consisting of a ?-lactam anti-infective (cefotaxime, ceftriaxone, ampicillin and sulbactam, piperacillin and tazobactam) and either a macrolide or a fluoroquinolone.
For inpatient treatment of severe CAP in patients hospitalized in an ICU, the ATS recommends that those not at risk for Ps. aeruginosa infection receive a 2-drug empiric regimen consisting of an IV b-lactam anti-infective (cefotaxime, ceftriaxone) and either an IV macrolide (azithromycin) or IV fluoroquinolone.
If risk factors for Ps. aeruginosa are present in patients with severe CAP admitted to an ICU, the ATS recommends an empiric regimen that includes 2 antipseudomonal agents and provides coverage for multidrug-resistant S. pneumonia and Legionella. Therefore, the ATS recommends that these patients receive a 2-drug empiric regimen that includes an IV antipseudomonal b-lactam anti-infective (e.g., cefepime, piperacillin and tazobactam, imipenem, meropenem) and an IV antipseudomonal fluoroquinolone (e.g., ciprofloxacin) or, alternatively, a 3-drug empiric regimen that includes one of the IV antipseudomonal ?-lactams, an IV aminoglycoside, and either an IV macrolide (e.g., azithromycin) or an IV nonpseudomonal quinolone.
Septicemia
Cefoperazone, cefotaxime, ceftazidime, ceftizoxime, and ceftriaxone are used parenterally for the treatment of bacteremia/septicemia caused by susceptible bacteria (e.g., S. aureus, S. pneumoniae, E. coli, H. influenzae, K. pneumoniae).
The choice of anti-infective agent for the treatment of sepsis syndrome should be based on the probable source of infection, gram-stained smears of appropriate clinical specimens, the immune status of the patient, and current patterns of bacterial resistance within the hospital and local community. Certain parenteral cephalosporins (i.e., cefepime, cefotaxime, ceftizoxime, ceftriaxone, ceftazidime) are good choices for the treatment of gram-negative sepsis.
Ceftazidime is less active against gram-positive cocci, and most cephalosporins (except cefepime and ceftazidime) have limited activity against Ps. aeruginosa. For the initial treatment of life-threatening sepsis in adults (unless presence of anaerobic bacteria, oxacillin-resistant staphylococci [previously known as methicillin-resistant staphylococci], or bacterial endocarditis is suspected), some clinicians suggest that use of a parenteral cephalosporin (i.e., cefepime, cefotaxime, ceftriaxone) given in conjunction with an aminoglycoside (amikacin, gentamicin, tobramycin) is one of several preferred regimens.
Some clinicians recommend use of vancomycin (alone or in conjunction with gentamicin and/or rifampin) when oxacillin-resistant staphylococci are a possible cause of sepsis; when bacterial endocarditis is suspected and therapy must be initiated before results of in vitro testing are available to identify the pathogen, a regimen of vancomycin and gentamicin can be used.
For treatment of suspected bacteremia in neutropenic patients, suggested regimens for initial therapy include cefepime, ceftazidime, imipenem, or meropenem used alone or, in seriously ill patients, used in conjunction with an aminoglycoside (amikacin, gentamicin, tobramycin).
A regimen of either ticarcillin and clavulanate potassium or piperacillin sodium and tazobactam sodium used in conjunction with amikacin may be equally effective.
Gonorrhea and Associated Infections
Cefoxitin, cefotaxime, ceftizoxime, ceftriaxone, and cefuroxime are used parenterally and cefixime, and cefpodoxime proxetil, and cefuroxime axetil are used orally for the treatment of uncomplicated gonorrhea caused by penicillinase-producing Neisseria gonorrhoeae (PPNG) or nonpenicillinase-producing N. gonorrhoeae. Cefoxitin, cefotaxime, ceftizoxime, ceftriaxone, and cefuroxime also are used parenterally for the treatment of disseminated gonorrhea and other gonococcal infections and oral cefixime is used for follow-up therapy after initial therapy with a parenteral cephalosporin for the treatment of disseminated gonococcal infections.
Ceftriaxone is considered a drug of choice for the treatment of most N. gonorrhoeae infections, including uncomplicated gonococcal infections of the cervix, urethra, rectum, and pharynx; disseminated gonococcal infections; gonococcal conjunctivitis; gonococcal meningitis and endocarditis; gonococcal epididymitis or proctitis in adults and adolescents; gonococcal ophthalmia neonatorum; and uncomplicated gonorrhea or disseminated gonococcal infections in neonates and children.
Ceftriaxone also is used in conjunction with other agents for empiric anti-infective prophylaxis in sexual assault victims.
The CDC states that, although other parenteral or oral cephalosporins may be as effective as ceftriaxone in the treatment of gonococcal infections, they do not appear to offer any clear advantage over ceftriaxone. Based on experience in adults, the AAP states that use of oral cefixime can be considered for the treatment of uncomplicated gonorrhea in young children provided that follow-up can be assured.
The CDC and many clinicians currently recommend that uncomplicated cervical, urethral, or rectal infections caused by penicillinase-producing N. gonorrhoeae (PPNG) or nonpenicillinase-producing N. gonorrhoeae in adults and adolescents be treated with a single IM dose of ceftriaxone, a single oral dose of cefixime, or a single oral dose of certain fluoroquinolones (ciprofloxacin, ofloxacin, levofloxacin) given in conjunction with an anti-infective regimen effective for presumptive treatment of chlamydia (e.g., a single dose of oral azithromycin or a 7-day regimen of oral doxycycline).
However, fluoroquinolones should not be used for the treatment of gonorrhea acquired in Asia or the pacific islands (including Hawaii) and may be inadvisable for infections acquired in other areas where N. gonorrhoeae with quinolone resistance have been reported (including California). (See Uses: Gonorrhea and Associated Infections, in Ciprofloxacin 8:12..)
Alternative regimens that are recommended by the CDC for the treatment of uncomplicated cervical, urethral, or rectal gonorrhea in adults and adolescents include a single IM dose of spectinomycin, a single IM dose of certain cephalosporins (ceftizoxime, cefotaxime, cefoxitin), or a single oral dose of certain fluoroquinolones (gatifloxacin, lomefloxacin, norfloxacin) given in conjunction with an anti-infective regimen effective for presumptive treatment of chlamydia. Uncomplicated pharyngeal gonococcal infections should be treated with a single IM dose of ceftriaxone or, alternatively, a single oral dose of ciprofloxacin given in conjunction with an anti-infective regimen effective for presumptive treatment of chlamydia.
For the treatment of disseminated gonococcal infections in adults and adolescents, the CDC and many clinicians recommend that therapy be initiated with a multiple-dose regimen of IM or IV ceftriaxone.
Alternative regimens recommended by the CDC for disseminated gonococcal infections include multiple-dose parenteral regimens of certain IV cephalosporins (cefotaxime, ceftizoxime), certain IV fluoroquinolones (ciprofloxacin, levofloxacin), or IM spectinomycin.
The initial parenteral regimen should be continued for 24-48 hours after improvement begins; therapy can then be switched to oral cefixime, oral ciprofloxacin, oral ofloxacin, or oral levofloxacin and continued to complete at least 1 week of treatment. Unless presence of coexisting chlamydial infection has been excluded by appropriate testing, individuals being treated for disseminated gonococcal infections should also receive an anti-infective regimen effective for presumptive treatment of chlamydia.
The CDC and AAP state that IV or IM ceftriaxone is the drug of choice for the treatment of gonococcal ophthalmia neonatorum or for parenteral prophylaxis in neonates born to women with documented, untreated gonococcal infection; the AAP recommends use of IV or IM cefotaxime as an alternative.
The CDC and AAP recommend that neonates and infants with localized gonococcal scalp abscesses or disseminated gonococcal infections should receive ceftriaxone or cefotaxime.
For the treatment of uncomplicated or disseminated gonococcal infections in prepubertal children who weigh less than 45 kg, the CDC and AAP generally recommend use of IM or IV ceftriaxone. Children weighing 45 kg or more generally can receive regimens recommended for adults and adolescents.
The CDC states that oral cephalosporins have not been adequately evaluated for the treatment of gonococcal infections in children. Gonorrhea frequently is associated with coexisting chlamydial and mycoplasmal infections; however, cephalosporins, penicillins, spectinomycin, and most single-dose quinolone regimens are ineffective in the treatment of these coexisting infections. Because of the risks associated with untreated coexisting chlamydial infections, the CDC and most clinicians currently state that therapy for uncomplicated gonorrhea or disseminated gonococcal infections should be given in conjunction with presumptive treatment for chlamydia.
For additional information on current recommendations for the treatment of gonorrhea and associated infections, see Uses: Gonorrhea and Associated Infections in Ceftriaxone 8:12.06.12.
Pelvic Inflammatory Disease
Several parenteral cephalosporins (cefotaxime, ceftizoxime, ceftriaxone) and closely related cephamycins (cefotetan, cefoxitin) have been used in the treatment of acute pelvic inflammatory disease (PID); these drugs are inactive against C. trachomatis and should not be used alone in the treatment of PID. PID is an acute or chronic inflammatory disorder in the upper female genital tract and can include any combination of endometritis, salpingitis, tubo-ovarian abscess, and pelvic peritonitis.
PID generally is a polymicrobial infection most frequently caused by N. gonorrhoeae and/or Chlamydia trachomatis; however, organisms that can be part of the normal vaginal flora (e.g., anaerobic bacteria, Gardnerella vaginalis, H. influenzae, enteric gram-negative bacilli, S. agalactiae) or mycoplasma (e.g., Mycoplasma hominis, Ureaplasma urealyticum) also may be involved. PID is treated with an empiric regimen that provides broad-spectrum coverage.
The regimen should be effective against N. gonorrhoeae and C. trachomatis and also probably should be effective against anaerobes, gram-negative facultative bacteria, and streptococci. The optimum empiric regimen for the treatment of PID has not been identified.
A wide variety of parenteral and oral regimens have been shown to achieve clinical and microbiologic cure in randomized studies with short-term follow-up; however, only limited data are available to date regarding elimination of infection in the endometrium and fallopian tubes or intermediate or long-term outcomes, including the impact of these regimens on the incidence of long-term sequelae of PID (e.g., tubal infertility, ectopic pregnancy, pain).
Although many clinicians have recommended that all patients with acute PID be hospitalized so that bedrest and supervised treatment with parenteral anti-infectives could be initiated, the CDC currently states that decisions regarding the necessity for hospitalization and whether an oral or parenteral regimen are most appropriate should be made on an individual basis since data are not available to date comparing efficacy of parenteral or oral therapy or inpatient or outpatient therapy.
Based on observational data and theoretical concerns, the CDC states that hospitalization is indicated if surgical emergencies such as appendicitis cannot be excluded; the patient is pregnant; the patient is unable to follow or tolerate an outpatient oral regimen; the patient has severe illness, nausea and vomiting, or high fever; the patient has a tuboovarian abscess; or a clinical response was not obtained with an oral anti-infective regimen.
Parenteral Regimens for PID
When a parenteral regimen is indicated for the treatment of patients with PID, the CDC and other clinicians generally recommend a 2-drug regimen of cefotetan (2 g IV every 12 hours) or cefoxitin (2 g IV every 6 hours) given in conjunction with doxycycline (100 mg IV or orally every 12 hours) or a 2-drug regimen of clindamycin (900 mg IV every 8 hours) and gentamicin (usually a 2-mg/kg IV or IM loading dose followed by 1.5 mg/kg every 8 hours).
While there is some evidence that certain parenteral cephalosporins (e.g., ceftizoxime, cefotaxime, ceftriaxone) also may be effective for the treatment of PID, the CDC states that there is less experience with use of these cephalosporins in patients with PID and these drugs may be less active than cefotetan or cefoxitin against anaerobic bacteria. The CDC states that limited data support the use of several alternative parenteral regimens for the treatment of acute PID, including IV levofloxacin (with or without IV metronidazole) or IV ampicillin sodium and sulbactam sodium with oral or IV doxycycline.
Traditionally, parenteral regimens for the treatment of PID have been continued for at least 48 hours after the patient demonstrates substantial clinical improvement and then an oral regimen continued to complete a total of 14 days of therapy; however, the CDC states that a transition to oral therapy may occur within 24 hours after the patient demonstrates clinical improvement and that decisions regarding such a transition should be guided by clinical experience. Most clinicians recommend at least 24 hours of direct inpatient observation for patients with tubo-ovarian abscesses, after which time anti-infective therapy at home is adequate.
Oral Regimens for PID
When PID is treated with an oral regimen, the CDC recommends a 14-day regimen that consists of oral ofloxacin (400 mg twice daily) or oral levofloxacin (500 mg once daily) with or without oral metronidazole (500 mg twice daily for 14 days) or a regimen that consists of a single dose of a parenteral cephalosporin (e.g., ceftriaxone, cefoxitin, ceftizoxime, cefotaxime) and a 14-day regimen of oral doxycycline with or without oral metronidazole (500 mg twice daily for 14 days).
Although ofloxacin is effective against both N. gonorrhoeae and C. trachomatis, the addition of metronidazole to the fluoroquinolone regimen may be necessary to provide adequate coverage against anaerobes. The optimal parenteral cephalosporin for the second regimen is unclear, although cefoxitin or ceftriaxone usually is preferred.
Data are not available to date regarding use of oral cephalosporins for the treatment of PID. There is evidence from clinical trials that a single dose of IM cefoxitin (given with probenecid) effectively produces a short-term clinical response in women with PID; however, because of theoretical limitations in cefoxitin’s coverage of anaerobes, the addition of metronidazole to the regimen may be necessary. In addition, metronidazole should be effective in the treatment of bacterial vaginosis, which is frequently associated with PID. There are limited data suggesting that use of oral doxycycline and oral metronidazole after primary parenteral therapy is safe and effective.
Patient Follow-up and Management of Sexual Partners
Regardless of whether an oral or parenteral regimen is used, patients with PID should demonstrate substantial clinical improvement (e.g., defervescence; reduction in direct or rebound abdominal tenderness; reduction in uterine, adnexal, and cervical motion tenderness) within 72 hours after initiation of anti-infective therapy, and patients being treated on an outpatient basis should receive a follow-up examination within this period to ensure that a response is obtained. If clinical improvement is not evident within 72 hours, hospitalization usually is required and additional diagnostic tests and/or surgical intervention indicated. In women who had documented infections with N. gonorrhoeae or C. trachomatis, some experts recommend rescreening for these organisms 4-6 weeks after therapy is completed.
Sexual partners of women with PID should be examined and treated if they had sexual contact during the 60 days preceding the onset of symptoms in the patients. Evaluation and treatment are imperative because of the risk for reinfection and the strong likelihood of urethral gonococcal or chlamydial infection in the partner. Male partners of women with PID caused by N. gonorrhoeae or C. trachomatis often are asymptomatic. Sex partners should be treated empirically with regimens effective against these organisms, regardless of the apparent etiology of PID or pathogens isolated from the infected woman.
Chancroid
Ceftriaxone is used for the treatment of genital ulcers caused by H. ducreyi (chancroid), and is considered a drug of choice for the treatment of this infection. (See Uses: Chancroid, in Ceftriaxone Sodium 8:12.06.12.)
Lyme Disease
Ceftriaxone, cefuroxime axetil, or cefotaxime is recommended by the IDSA, AAP, and other clinicians for the treatment of Lyme disease. Lyme disease (Lyme borreliosis) is a spirochetal disease caused by Borrelia burgdorferi. Anti-infective therapy usually is effective in all stages of the disease, and appropriate treatment of early Lyme disease shortens the duration of symptoms and generally prevents the development of late sequelae. (See Treatment of Early Localized or Disseminated Lyme Disease under Spirochetal Infections: Lyme Disease, in Uses in the Tetracyclines General Statement 8:12.24.)
Diagnostic Considerations
Diagnosis of Lyme disease is based principally on clinical findings, and treating patients with early disease solely on the basis of objective signs and a known exposure often is appropriate. Individuals with known endemic exposure to B. burgdorferi and clinician-diagnosed erythema migrans can receive treatment for Lyme disease without serologic testing; however, erythema migrans should be clinically differentiated from similar rashes that are not caused by B. burgdorferi infection. In areas of low or no endemic risk, the likelihood of Lyme disease in a patient with a rash resembling erythema migrans is low. Serologic testing can provide valuable supportive diagnostic information in patients with endemic exposure and objective clinical findings that indicate later stage disseminated Lyme disease.
Negative test results are useful in ruling out Lyme disease in patients with clinical findings compatible with disseminated or late-stage infection. Since the proportion of false-positive test results increases when the pretest probability of Lyme disease is low, the use of testing to make a diagnosis of Lyme disease in individuals without endemic exposure is not recommended. The CDC and National Institute of Allergy and Infectious Diseases (NIAID) state that clinicians should be familiar with current recommendations for diagnosis and treatment of Lyme disease and should be alert for and know how to minimize potential complications associated with therapy for the disease.
When serologic testing is indicated to aid in diagnosis, the CDC and Association of State, Territorial, and Public Health Laboratory Directors (ASTPHLD), and other clinicians recommend initial testing with a sensitive screening test, either an enzyme-linked immunosorbent assay (ELISA) or an indirect fluorescent antibody (IFA) test, followed by testing with the more specific Western blot (immunoblot) test to corroborate equivocal or positive results obtained with the initial test.
Although anti-infective treatment in early localized disease may blunt or abrogate the antibody response, patients with early disseminated or late-stage disease usually have strong serologic reactivity and demonstrate expanded IgG Western blot banding patterns to diagnostic B. burgdorferi antigens. Antibodies often persist for months or years following successfully treated or untreated infection. Therefore, seroreactivity alone cannot be used as a marker of active disease. Repeated infection with B. burgdorferi has been reported, and neither a positive serologic test result and/or a history of prior Lyme disease ensures that an individual has protective immunity.
Treatment of Early Localized or Disseminated Disease
The IDSA, AAP, and other clinicians recommend the use of oral doxycycline or oral amoxicillin as first-line therapy for the treatment of early localized or early disseminated Lyme disease associated with erythema migrans, in the absence of neurologic involvement or third-degree atrioventricular (AV) heart block. Cefuroxime axetil is an effective alternative agent that, because of its greater cost, is recommended for patients with early Lyme disease who are allergic to or intolerant of doxycycline and amoxicillin. Although the optimal duration of therapy has not been established, most clinicians treat early Lyme disease for 14-21 days. Available evidence suggests that first generation cephalosporins (e.g., cephalexin) are not effective for Lyme disease.
Oral doxycycline, amoxicillin, or cefuroxime axetil may be preferred to other drugs such as oral tetracycline hydrochloride, oral penicillin G, or penicillin V, particularly in patients with early disseminated infection, because of improved microbiologic activity, better GI absorption and tolerance, and/or higher CSF drug concentrations.
For more information on the efficacy of various anti-infective regimens in patients with early Lyme disease, see Treatment of Early Localized or Disseminated Lyme Disease under Spirochetal Infections: Lyme Disease, in Uses in the Tetracyclines General Statement 8:12.24. Transplacental transmission of B. burgdorferi appears to occur rarely, if at all, and epidemiologic studies in pregnant women have not documented an association between exposure to Lyme disease prior to conception or during pregnancy and subsequent fetal death, congenital malformations, or prematurity.
The IDSA, AAP, and other clinicians state that pregnant or nursing women need not be treated differently than other patients with Lyme disease, except that they should not receive tetracyclines.
Although oral anti-infectives (e.g., doxycycline, amoxicillin, cefuroxime axetil) generally are effective for the treatment of the early stages of the disease (e.g., erythema migrans, isolated facial nerve palsy, mild arthritis or carditis), more serious manifestations associated with early disseminated or late disease (e.g., meningitis, radiculoneuritis, severe carditis) require higher dosage, more prolonged therapy, and/or parenteral anti-infectives (e.g., IV ceftriaxone, IV cefotaxime, or IV penicillin G for 2-4 weeks). (See Lyme Disease in Uses: Spirochetal Infections, in the Natural Penicillins General Statement 8:12.16.04 and see Lyme Disease in Uses: Spirochetal Infections, in Ceftriaxone 8:12.06.12.)
While evidence supporting the superiority of IV versus oral therapy currently is unavailable, most clinicians recommend that patients with severe cardiac involvement receive ceftriaxone, cefotaxime, or penicillin G IV for 14-28 days. Some clinicians also recommend use of these IV regimens in patients with first-degree AV block and a PR-interval greater than 0.3 seconds. The IDSA and other clinicians recommend that patients with third-degree AV heart block be hospitalized for cardiac monitoring because of the potential for life-threatening complications.
Patients with third-degree AV block may require a temporary pacemaker. While patients with uncomplicated Lyme arthritis generally can be treated with a prolonged course (e.g., 28 days) of oral anti-infectives (i.e., doxycycline or amoxicillin), patients with Lyme arthritis and associated neurologic disease documented by CSF analysis should receive IV ceftriaxone or cefotaxime for 14-28 days; alternative therapy is IV penicillin G. Some clinicians state that IV ceftriaxone may be preferable to IV penicillin G for serious manifestations of early disseminated or late Lyme disease (i.e., those involving major organs) based on ceftriaxone’s greater in vitro and in vivo activity against B. burgdorferi, excellent CSF penetration, and prolonged serum concentrations achievable with once-daily administration of the drug.. (See Lyme Disease in Uses: Spirochetal Infections, in Ceftriaxone 8:12.06..)
The IDSA and other clinicians state that IV cefotaxime is an effective alternative to ceftriaxone or IV penicillin G in patients requiring parenteral anti-infective therapy for Lyme disease; while cefotaxime must be administered 3-4 times daily (compared with once-daily administration of ceftriaxone), cefotaxime has not been associated with the biliary complications reported with IV ceftriaxone. (See Cautions: GI Effects, in Ceftriaxone 8:12.06..)
Treatment of Late Disease
Comparative studies evaluating different antibiotic regimens in patients with late Lyme disease generally are lacking. The IDSA and other clinicians recommend 14-28 days of therapy with IV ceftriaxone or a 28-day course of a recommended oral anti-infective in patients who have persistent or recurrent joint swelling after receiving an initial recommended antibiotic regimen. However, clinicians should consider allowing several months for joint inflammation to resolve after initial treatment before an additional course of anti-infective therapy is given. Patients with late neurologic disease affecting the CNS or peripheral nervous system (e.g., encephalopathy, neuropathy) should receive 14-28 days of IV ceftriaxone; alternative therapy is IV cefotaxime or IV penicillin G. For additional details on anti-infective therapy in patients with late Lyme disease, see Treatment of Late or Persistent Manifestations of Lyme Disease under Spirochetal Infections: Lyme Disease, in Uses in the Tetracyclines General Statement 8:12.24.
Chronic Manifestations of Lyme Disease
Diagnosis of Lyme disease often is based on clinical manifestations, history of exposure in an endemic area, and positive results of an enzyme-linked immunoassay for serum antibodies to the organism. However, because of assay variability, lack of assay standardization, and the potential for false-positive results, this assay alone does not provide a definitive diagnosis of the disease.
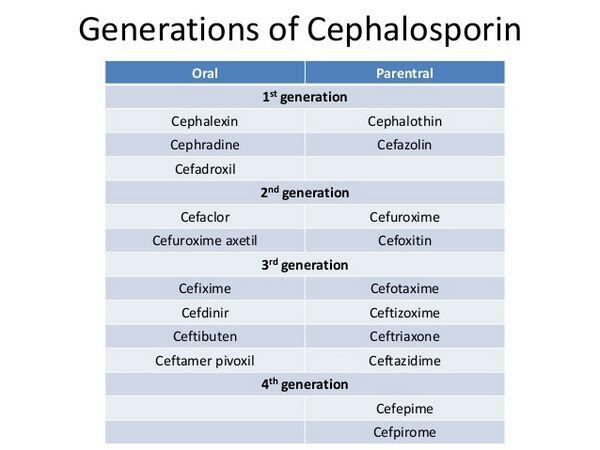
After receiving recommended antibiotic therapy for Lyme disease, some patients manifest a post-infectious syndrome, which some clinicians have referred to as chronic Lyme disease or post-Lyme disease syndrome. (See Chronic Lyme Disease or Post-Lyme Disease Syndrome under Spirochetal Infections: Lyme Disease, in Uses in the Tetracyclines General Statement 8:12.24.)
Many patients with positive antibody test results but without classic Lyme disease manifestations (e.g., erythema migrans) reportedly have received empiric IV antibiotic therapy for suspected, late-stage Lyme disease; some of these patients subsequently have been diagnosed as having fibromyalgia or chronic fatigue syndrome, which reportedly can be triggered in some cases by B. burgdorferi infection.
Although case reports and uncontrolled studies have reported benefit from prolonged antibiotic therapy in patients with chronic manifestations of Lyme disease, the IDSA and other clinicians state that currently available evidence is insufficient to consider chronic Lyme disease a separate diagnostic entity 329, 666 and that no controlled clinical studies currently support the efficacy of repeated or prolonged courses of oral and/or IV antibiotics in patients who remain symptomatic after receiving appropriate therapy for Lyme disease (i.e., 2-4 weeks of recommended anti-infective treatment).
Results of 2 case-control studies in patients with primary or secondary diagnosis of Lyme disease demonstrated that most patients hospitalized for suspected Lyme disease lacked documented objective manifestations of disseminated disease or seropositivity to B. burgdorferi and did not achieve satisfactory remission of their symptoms despite repeated and often prolonged courses of anti-infectives (e.g., with ceftriaxone). In addition, anti-infective therapy for unsubstantiated Lyme disease in these and other studies has been associated with serious complications (e.g., biliary disease resulting in cholecystectomy) in some patients; at least one death has been reported in a patient receiving prolonged empiric therapy with IV antibiotics.(See Cautions: GI Effects, in Ceftriaxone 8:12.06..)
The American College of Rheumatology and IDSA state that the risks and costs of treating suspected Lyme disease empirically with IV antibiotics (e.g. ceftriaxone) exceed the benefits in patients with a positive antibody titer for Borrelia burgdorferi and only nonspecific complaints of myalgia or fatigue.
Duration of Therapy
Although the optimum duration of therapy for Lyme disease has not been definitely established, more than 4 weeks of anti-infective therapy for the disease is not generally indicated.
Empiric Therapy in Febrile Neutropenic Patients
Cefepime, ceftazidime, and ceftriaxone are used parenterally for empiric anti-infective therapy of presumed bacterial infections in febrile neutropenic adults or pediatric patients. Cefepime and ceftazidime have been effective when used as monotherapy for empiric therapy in febrile neutropenic patients, but the drugs also have been used in combination regimens.
Results of several studies in febrile granulocytopenic patients indicate that ceftazidime used alone may be as effective as combination regimens that include ceftazidime and an aminoglycoside (e.g., amikacin, gentamicin, tobramycin) or combination regimens that include some other b-lactam antibiotic (e.g., cefepime, ceftriaxone) and an aminoglycoside for empiric therapy in these patients.
Because gram-positive bacteria are being reported with increasing frequency in febrile granulocytopenic patients and because ceftazidime is less active against these organisms than many other cephalosporins and β-lactam antibiotics, some clinicians suggest that an anti-infective agent active against staphylococci (e.g., vancomycin) probably should be used concomitantly if ceftazidime is used for empiric therapy in these patients. Unlike ceftazidime, other anti-infectives used for empiric therapy (e.g., cefepime, imipenem, meropenem) have good activity against viridans streptococci and S. pneumoniae and there is some evidence that concomitant use of vancomycin is required less frequently with cefepime than with ceftazidime.
Other clinicians suggest that ceftazidime alone may be adequate for initial empiric therapy in some institutions, provided that additional anti-infective therapy (e.g., vancomycin) is added promptly if ceftazidime-resistant organisms are identified. While ceftriaxone has been used alone for empiric therapy in some febrile neutropenic patients considered to be at low risk, use of ceftriaxone monotherapy may not provide adequate coverage against some potential pathogens (e.g., Ps. aeruginosa) and generally is not recommended for empiric therapy in febrile neutropenic patients.
Successful treatment of infections in granulocytopenic patients requires prompt initiation of empiric anti-infective therapy (even when fever is the only sign or symptom of infection) and appropriate modification of the initial regimen if the duration of fever and neutropenia is protracted, if a specific site of infection is identified, or if organisms resistant to the initial regimen are present.
The initial empiric regimen should be chosen based on the underlying disease and other host factors that may affect the degree of risk and on local epidemiologic data regarding usual pathogens in these patients and data regarding their in vitro susceptibility to available anti-infective agents.
The fact that gram-positive bacteria have become a predominant pathogen in febrile neutropenic patients should be considered when selecting an empiric anti-infective regimen. No empiric regimen has been identified that would be appropriate for initial treatment of all febrile neutropenic patients.
Regimens that have been recommended for empiric therapy in febrile neutropenic patients with presumed bacterial infections include monotherapy with a third or fourth generation cephalosporin (i.e., ceftazidime, cefepime) or a carbapenem (e.g., imipenem and cilastatin sodium, meropenem); combination therapy consisting of a b-lactam antibiotic (e.g., ceftazidime, ceftriaxone), a carbapenem (e.g., imipenem, meropenem), an extended-spectrum penicillin (e.g., ticarcillin, piperacillin [no longer commercially available in the US]), or a fixed combination of an extended-spectrum penicillin and a b-lactamase inhibitor (e.g., piperacillin sodium and tazobactam sodium, ticarcillin disodium and clavulanate potassium) given in conjunction with an aminoglycoside (e.g., amikacin, gentamicin, tobramycin).
The IDSA recommends use of a parenteral empiric regimen in most febrile neutropenic patients; use of an oral regimen (e.g., oral ciprofloxacin and oral amoxicillin and clavulanate) should only be considered in selected adults at low risk for complications who have no focus of bacterial infection and no signs or symptoms of systemic infection other than fever. At health-care facilities where gram-positive bacteria are common causes of serious infection and use of vancomycin in the initial empiric regimen is considered necessary, the IDSA recommends 2- or 3-drug combination therapy that includes vancomycin and either cefepime, ceftazidime, imipenem, or meropenem given with or without an aminoglycoside; vancomycin should be discontinued 24-48 hours later if a susceptible gram-positive bacterial infection is not identified.
At health-care facilities where vancomycin is not indicated in the initial empiric regimen, the IDSA recommends monotherapy with a third or fourth generation cephalosporin (ceftazidime, cefepime) or a carbapenem (imipenem, meropenem) for uncomplicated cases; however, for complicated cases or if anti-infective resistance is a problem, combination therapy consisting of an aminoglycoside (amikacin, gentamicin, tobramycin) given in conjunction with an antipseudomonal penicillin (ticarcillin and clavulanate, piperacillin and tazobactam), an antipseudomonal cephalosporin (cefepime, ceftazidime), or a carbapenem (imipenem, meropenem) is recommended.
Regardless of the initial regimen selected, patients should be reassessed after 3-5 days of treatment and the anti-infective regimen altered (if indicated) based on the presence or absence of fever, identification of the causative organism, and the clinical condition of the patient. Published protocols for the treatment of infections in febrile neutropenic patients should be consulted for specific recommendations regarding selection of the initial empiric regimen, when to change the initial regimen, possible subsequent regimens, and duration of therapy in these patients. In addition, consultation with an infectious disease expert knowledgeable about infections in immunocompromised patients is advised.
Prevention of Perinatal Group B Streptococcal Disease
Cefazolin is used as an alternative to penicillin G or ampicillin for prevention of perinatal group B streptococcal (GBS) disease. Pregnant women who are colonized with GBS in the genital or rectal areas can transmit GBS infection to their infants during labor and delivery resulting in invasive neonatal infection that can be associated with substantial morbidity and mortality. Intrapartum anti-infective prophylaxis for prevention of early-onset neonatal GBS disease is administered selectively to women at high risk for transmitting GBS infection to their neonates.
When intrapartum prophylaxis is indicated in the mother, penicillin G (5 million units IV initially followed by 2.5 million units IV every 4 hours until delivery) is the regimen of choice and ampicillin (2 g IV initially followed by 1 g IV every 4 hours until delivery) is the preferred alternative. When intrapartum prophylaxis to prevent GBS in the neonate is indicated in women who are hypersensitive to penicillins, a regimen of IV clindamycin (900 mg IV every 8 hours until delivery) or IV erythromycin (500 mg IV every 6 hours until delivery) is recommended for those allergic to penicillins who are at high risk for anaphylaxis (e.g., those with a history of immediate penicillin hypersensitivity, such as anaphylaxis, angioedema, or urticaria; those with a history of asthma or other conditions that would make anaphylaxis more dangerous or difficult to treat, including individuals receiving ?adrenergic blocking agents).
For those allergic to penicillins who are not at high risk for anaphylaxis, the CDC states that a regimen of IV cefazolin (2 g IV initially followed by 1 g IV every 8 hours until delivery) should be used since this cephalosporin has a narrow spectrum of activity and is associated with high intraamniotic concentrations. The fact that GBS with in vitro resistance to clindamycin and erythromycin have been reported with increasing frequency should be considered when choosing an alternative to penicillins. Vancomycin should be used for intrapartum prophylaxis of perinatal GBS disease only in women with penicillin allergy who are at high risk for anaphylaxis and only when GBS isolates are known or likely to be resistant to clindamycin and erythromycin. For additional information on prevention of perinatal group B streptococcal disease, see Uses: Prevention of Perinatal Group B Streptococcal Disease, in the Natural Penicillins General Statement 8:12.16.04.
Perioperative Prophylaxis
Cefazolin, cefotaxime, ceftriaxone, and cefuroxime have been used perioperatively to reduce the incidence of infections in patients undergoing GI or genitourinary tract surgery, obstetric and gynecologic surgery (e.g., abdominal or vaginal hysterectomy, cesarean section), cardiovascular surgery, noncardiac thoracic surgery or prosthetic arthroplasty.
There is evidence that perioperative prophylaxis with an appropriate anti-infective agent can decrease the incidence of infection, particularly wound infection, after certain procedures; however, the benefits of prophylaxis must be weighed against the risks of adverse effects (including sensitivity reactions), emergence of resistant bacteria or superinfection, drug interactions, and cost.
Therefore, perioperative prophylaxis usually is recommended only for procedures with a high rate of infection, procedures involving implantation of prosthetic material, and procedures in which the consequences of infection are especially serious.
Because cefazolin has a narrow spectrum of activity and is active against staphylococci and streptococci, has a moderately long serum half-life, and has been shown to be effective, it is considered by many clinicians to be the drug of choice for perioperative prophylaxis for a variety of procedures, including cardiac, noncardiac thoracic, esophageal, gastroduodenal, biliary tract, gynecologic and obstetric, head and neck, neurologic, orthopedic, and vascular surgeries.
Cefuroxime also is considered a drug of choice for cardiac surgery and noncardiac thoracic surgery. However, a drug or regimen with activity against anaerobic bacteria (e.g., cefotetan or cefoxitin with or without gentamicin; cefazolin and metronidazole; clindamycin with or without gentamicin) generally is preferred for surgery involving the distal intestinal tract or ruptured viscus, including colorectal surgery and appendectomy.
There is no evidence that third generation cephalosporins are more effective than first or second generation cephalosporins (e.g., cefazolin, cefuroxime) for perioperative prophylaxis in patients undergoing obstetric and gynecologic, biliary tract, cardiovascular, or orthopedic surgery. Some clinicians state that third generation cephalosporins (e.g., cefoperazone, cefotaxime, ceftriaxone, ceftazidime, ceftizoxime) or fourth generation cephalosporins (e.g., cefepime) should not be used for perioperative prophylaxis since they are expensive, some are less active than cefazolin against staphylococci, they have a spectrum of activity that is wider than necessary for organisms encountered in elective surgery, and their use for prophylaxis promotes emergence of resistant organisms.
Cardiac Surgery
Perioperative prophylaxis can decrease the incidence of infection after cardiac surgery.
Results of a pooled analysis of 7 placebo-controlled studies indicate that perioperative prophylaxis reduces the incidence of infection after permanent pacemaker implantation. For perioperative prophylaxis in patients undergoing cardiac surgery (e.g., prosthetic valve, coronary artery bypass, other open-heart surgery, pacemaker or defibrillator implant), many clinicians recommend IV cefazolin or IV cefuroxime sodium. Although routine use of vancomycin for perioperative prophylaxis is not recommended, vancomycin may be considered an alternative for perioperative prophylaxis in cardiac surgery patients hypersensitive to ?-lactam anti-infectives or at institutions where oxacillin-resistant S. aureus and S. epidermidis frequently cause wound infection.
Noncardiac Thoracic Surgery
Although studies evaluating use of perioperative prophylaxis in patients undergoing pulmonary surgery are sparse, such prophylaxis is routinely used. In one randomized, double-blind, placebo-controlled study in patients undergoing noncardiac thoracic surgery, a single preoperative dose of cefazolin decreased the incidence of postoperative surgical site infection, but did not affect the incidence of postoperative empyema or nosocomial pneumonia.
There is evidence from a placebo-controlled study that multiple doses of a cephalosporin (cefazolin) can prevent infection after closed-tube thoracostomy for chest trauma; however, some clinicians suggest that insertion of chest tubes for other indications, such as spontaneous pneumothorax, does not require prophylaxis.
For perioperative prophylaxis in patients undergoing noncardiac thoracic surgery, many clinicians recommend IV cefazolin or IV cefuroxime sodium. Although routine use of vancomycin for perioperative prophylaxis is not recommended, vancomycin may be considered an alternative for perioperative prophylaxis in noncardiac thoracic surgery patients who are hypersensitive to β-lactam anti-infectives or at institutions where oxacillin-resistant S. aureus and S. epidermidis frequently cause wound infection.
GI Surgery
Esophageal and Gastroduodenal Surgery
Perioperative prophylaxis generally is not indicated for patients undergoing routine gastroesophageal endoscopy, but some clinicians recommend such prophylaxis in patients undergoing placement of percutaneous gastrostomy and for high-risk patients undergoing esophageal dilation or sclerotherapy of varices. Perioperative prophylaxis usually is recommended for esophageal surgery in the presence of obstruction since the risk of infection is increased in these patients.
Perioperative prophylaxis can decrease the risk of postoperative infection after gastroduodenal surgery in patients who are at high risk of infection because they are morbidly obese or because they have decreased gastric acidity or decreased GI motility as the result of obstruction, hemorrhage, gastric ulcer, malignancy, or therapy with a histamine H2-receptor antagonist (e.g., ranitidine) or a proton pump inhibitor (e.g., omeprazole).
For perioperative prophylaxis in high-risk patients undergoing esophageal or gastroduodenal surgery (e.g., those who are morbidly obese or have esophageal obstruction, decreased gastric acidity or decreased GI motility), many clinicians recommend IV cefazolin as the drug of choice; however, some clinicians prefer cefoxitin in these patients since it provides better coverage against anaerobic bacteria.
Biliary Tract Surgery
Many clinicians recommend perioperative prophylaxis for patients undergoing biliary tract surgery who are at high risk of infection (e.g., those older than 70 years of age or those with acute cholecystitis, a nonfunctioning gallbladder, obstructive jaundice, or common duct stones). While many clinicians recommend perioperative prophylaxis for high-risk patients undergoing endoscopic retrograde cholangiopancreatography (ERCP), some clinicians state that such prophylaxis is not necessary for low-risk patients undergoing elective laparoscopic cholecystectomy since there is no evidence that such prophylaxis provides any benefit in these patients.
For perioperative prophylaxis in high-risk patients undergoing biliary tract surgery (e.g., those older than 70 years of age or those with acute cholecystitis, nonfunctioning gallbladder, obstructive jaundice, or common duct stones), many clinicians recommend IV cefazolin as the drug of choice; however, some clinicians prefer cefoxitin in these patients since it provides better coverage against anaerobic bacteria.
Colorectal Surgery
There is evidence that perioperative prophylaxis can decrease the incidence of infection after colorectal surgery, and such prophylaxis usually is recommended. For perioperative prophylaxis in patients undergoing colorectal surgery, many clinicians recommend a regimen of IV cefotetan or IV cefoxitin; a regimen of IV cefazolin and IV metronidazole; or a regimen of oral erythromycin and oral neomycin. It has been suggested that the oral regimen may be as effective as the parenteral regimens for patients undergoing elective colorectal surgery.
Many clinicians use both the oral regimen and a parenteral regimen for perioperative prophylaxis in patients undergoing colorectal surgery; however, it is unclear whether this combined regimen is more effective than use of either an oral or parenteral regimen alone. In a randomized, prospective study in patients undergoing elective colorectal surgery, the overall incidence of intra-abdominal septic complications in those who received mechanical bowel preparation and an oral regimen (erythromycin and neomycin) alone was similar to that in those who received both the oral regimen and a parenteral regimen (cefoxitin); however, the incidence of abdominal wound infection was higher in those who received the oral regimen alone (14.%) than in those who received the combined oral and parenteral regimen (5%).
Appendectomy
There is evidence that perioperative prophylaxis can reduce the incidence of infection after surgery for acute appendicitis, and some clinicians recommend use of IV cefotetan or IV cefoxitin for perioperative prophylaxis in patients undergoing nonperforated appendectomy. If perforation has occurred, anti-infectives are considered treatment rather than prophylaxis and are continued postoperatively as long as clinically indicated. (See Contaminated Surgery under Uses: Perioperative Prophylaxis.)
Gynecologic and Obstetric Surgery
Perioperative prophylaxis decreases the incidence of infection after vaginal and abdominal hysterectomy. In addition, there is evidence that perioperative prophylaxis can prevent infection after emergency cesarean section in high-risk situations (e.g., active labor or premature rupture of membranes), after second trimester abortions, or after first trimester abortion in high-risk women. A pooled analysis of results of randomized, placebo-controlled studies in women who underwent therapeutic abortion before 16 weeks’ gestation indicates that perioperative prophylaxis reduces the overall risk of postabortal infection by 42% compared with placebo.
Many clinicians suggest that the preferred agents for perioperative prophylaxis in women undergoing vaginal or abdominal hysterectomy are IV cefazolin, IV cefotetan, or IV cefoxitin; IV cefazolin generally is the preferred agent for prophylaxis in women undergoing high-risk cesarean section or second trimester abortion, and IV penicillin G or oral doxycycline is preferred for high-risk first trimester abortions (e.g., previous history of PID, previous history of gonorrhea, multiple sexual partners).
Head and Neck Surgery
There is evidence that perioperative prophylaxis has decreased the incidence of surgical site infection after head and neck operations involving incisions through the oral or pharyngeal mucosa. For perioperative prophylaxis in patients undergoing head or neck surgery, many clinicians recommend a regimen of clindamycin and gentamicin or, alternatively, cefazolin.
Neurosurgery
Conflicting results have been reported in studies evaluating perioperative prophylaxis in patients undergoing implantation of permanent CSF shunts. There is evidence that use of an anti-infective agent active against staphylococci can decrease the incidence of infection after craniotomy. In patients undergoing spinal surgery, the postoperative infection rate after conventional lumbar discectomy is low and use of anti-infective agents has not been shown to be effective.
However, the risk of infection is higher after spinal procedures involving fusion, prolonged spinal surgery, or insertion of foreign material, and perioperative prophylaxis often is used in these patients despite the lack of controlled clinical studies demonstrating efficacy of such prophylaxis.
Despite the low risk of infection in other neurosurgical procedures, many clinicians use perioperative prophylaxis because of the serious consequences of surgical site infection in these patients. For perioperative prophylaxis in patients undergoing neurosurgery (e.g., craniotomy), many clinicians recommend use of IV cefazolin or, alternatively, IV vancomycin. Although routine use of vancomycin for perioperative prophylaxis is not recommended, vancomycin may be considered an alternative for perioperative prophylaxis in neurosurgery patients hypersensitive to ?-lactam anti-infectives or at institutions where oxacilllin-resistant S. aureus and S. epidermidis frequently cause wound infection.
Ophthalmic Surgery
Data are limited regarding the effectiveness of anti-infective prophylaxis in patients undergoing ophthalmic surgery; however, such prophylaxis is recommended by many clinicians since postoperative endophthalmitis can be devastating. Some clinicians state that there is no evidence that perioperative prophylaxis is needed for procedures that do not invade the globe. While there is no consensus regarding the most effective drug, route, or duration of perioperative prophylaxis in patients undergoing ophthalmic surgery, many clinicians recommend use of topical anti-infectives (e.g., gentamicin; tobramycin; ciprofloxacin; ofloxacin; neomycin and polymyxin b sulfates and gramicidin); some clinicians also recommend subconjunctival injection of an anti-infective agent (e.g., cefazolin).
Orthopedic Surgery
There is evidence that perioperative prophylaxis with antistaphylococcal agents given preoperatively can decrease the incidence of both early and late infection in patients undergoing joint replacement and can decrease the rate of infection in compound or open fracture and when hip and other fractures are treated with internal fixation by nails, plates, screws, or wires.
Results of a randomized study indicate that a single dose of a cephalosporin is more effective than placebo in preventing wound infection after surgical repair of closed fractures.
Results of a prospective, randomized study in patients undergoing diagnostic and operative arthroscopic surgery indicate that perioperative prophylaxis is not indicated in patients undergoing these procedures.
For perioperative prophylaxis in patients undergoing orthopedic surgery (e.g., total joint replacement, internal fixation of fractures), many clinicians recommend use of IV cefazolin or, alternatively, IV vancomycin.
Although routine use of vancomycin for perioperative prophylaxis is not recommended, vancomycin may be considered an alternative for perioperative prophylaxis in orthopedic surgery patients hypersensitive to β-lactam anti-infectives or at institutions where oxacillin-resistant S. aureus and S. epidermidis frequently cause wound infection.
Vascular Surgery
There is evidence that perioperative prophylaxis with a cephalosporin can decrease the incidence of postoperative surgical site infection after arterial reconstructive surgery on the abdominal aorta, vascular operations on the leg that include a groin incision, or amputation of the lower extremity for ischemia.
Many clinicians also recommend perioperative prophylaxis in patients undergoing implantation of any vascular prosthetic material (e.g., grafts for vascular access in hemodialysis); however, such prophylaxis generally is not indicated for carotid endarterectomy or brachial artery repair without prosthetic material.
For perioperative prophylaxis in patients undergoing vascular surgery (e.g., arterial surgery involving a prosthesis, the abdominal aorta, or a groin incision; lower extremity amputation for ischemia), many clinicians recommend use of IV cefazolin; however, some clinicians prefer cefoxitin in patients undergoing lower extremity amputation for ischemia since it provides better coverage against anaerobic bacteria.
Although routine use of vancomycin for perioperative prophylaxis is not recommended, vancomycin may be considered an alternative for perioperative prophylaxis in vascular surgery patients hypersensitive to β-lactam anti-infectives or at institutions where oxacillin-resistant S. aureus and S. epidermidis frequently cause wound infection.
Timing and Number of Doses
When perioperative prophylaxis is indicated in patients undergoing clean-contaminated or potentially contaminated surgery, administration of the anti-infective should be timed to ensure that bactericidal concentrations of the drug are established in serum and tissues by the time the initial surgical incision is made; therapeutic concentrations of the drug should then be maintained in serum and tissues throughout the operation and until, at most, a few hours after the incision is closed.
In most instances, a single IV dose given no more than 30 minutes before the incision provides adequate tissue concentrations throughout the procedure. If surgery is prolonged (more than 4 hours), major blood loss occurs, or an anti-infective with a short half-life (e.g., cefoxitin) is used, it may be advisable to administer one or more additional doses during the procedure; for prolonged procedures, some clinicians suggest that intraoperative doses be administered every 4-8 hours for the duration of the procedure. Although anti-infective prophylaxis regimens reported in published studies often include 1 or 2 postoperative doses in addition to the preoperative dose, many clinicians state that postoperative doses generally are unnecessary. Continuation of prophylaxis after surgery appears to be of no additional value and may increase the risk of toxicity and bacterial superinfection. If signs of infection occur following surgery, specimens should be obtained for identification of the causative organism and appropriate therapy instituted.
Contaminated Surgery
Patients undergoing contaminated or dirty surgery, such as that involving a perforated abdominal viscus, a compound fracture, a traumatic wound, or a laceration due to an animal or human bite are at risk for infection. When used in patients undergoing these procedures, anti-infective therapy is considered treatment rather than prophylaxis and is continued postoperatively for about 5 days.
Some clinicians recommend use of a regimen of IV cefoxitin or IV cefotetan (with or without IV gentamicin) or, alternatively, a regimen of IV clindamycin and IV gentamicin for patients undergoing surgery involving a ruptured viscus; if ruptured viscus occurs in a postoperative setting (dehiscence), anti-infective coverage of nosocomial pathogens should be included.
Cefazolin generally is the preferred anti-infective in patients undergoing surgery involving a traumatic wound; however, if a bite wound is involved, an anti-infective against anaerobes (e.g., amoxicillin and clavulanate potassium or ampicillin sodium and sulbactam sodium) should be used. For penetrating intracranial wounds, including gunshot injuries, a broad-spectrum anti-infective such as ampicillin sodium and sulbactam sodium is recommended.
Administration
Cephalosporins, in appropriate forms, may be administered orally, IV, by deep IM injection, or intraperitoneally. In general, orally administered cephalosporins should not be relied on for the treatment of the initial phase of severe infections or in patients with nausea and vomiting.
Cephalosporins should be given IV in patients with meningitis, septicemia, endocarditis, or other severe or life-threatening infections.
Cephalosporins have been administered by regional perfusion, subconjunctivally, intraventricularly, or intrathecally, but the risk of CNS toxicity must be considered with the latter route. (See Cautions: Other Adverse Effects.)
Dosage
The duration of cephalosporin therapy depends on the type of infection. Generally, therapy should be continued for a minimum of 48-72 hours after the patient becomes asymptomatic or evidence of eradication of the infection has been obtained. In infections caused by b-hemolytic streptococci, therapy should be continued for at least 10 days. At least 4-6 weeks of therapy may be required in serious infections such as septicemia, endocarditis, or osteomyelitis.
Dosage in Renal Impairment
In patients with impaired renal function, decreases in doses and/or frequency of administration of cephalosporins may be required and should be based on the degree of renal impairment, severity of the infection, susceptibility of the causative organism, and serum concentrations of the cephalosporins.
Cautions
Cephalosporins generally are well tolerated and with only a few exceptions, adverse effects reported with the various cephalosporin derivatives are similar. The most frequent adverse effects reported with oral cephalosporins include GI effects (diarrhea, nausea, vomiting), headache, and rash and the most frequent adverse effects reported with parenteral cephalosporins include local reactions at the injection site, adverse GI effects, and adverse hematologic effects.
Although not reported with other cephalosporins, derivatives that contain an N-methylthiotetrazole (NMTT) side chain (e.g., cefamandole [no longer commercially available in the US], cefoperazone, cefotetan) have been associated with an increased risk of hypoprothrombinemia and disulfiram-like reactions. In addition, while the clinical importance is unclear, derivatives that contain an NMTT side chain have caused adverse testicular effects in animal studies.
Dermatologic and Sensitivity Reactions
Hypersensitivity reactions have been reported in approximately 5% or less of patients receiving a cephalosporin.
These reactions include urticaria, pruritus, rash (maculopapular, erythematous, or morbilliform), fever and chills, eosinophilia, joint pain or inflammation, edema, facial edema, erythema, genital and anal pruritus, angioedema, shock, hypotension, vasodilatation, Stevens-Johnson syndrome, erythema multiforme, toxic epidermal necrolysis, and exfoliative dermatitis. Anaphylaxis, including a few fatalities, has occurred rarely with cephalosporins.
Serum sickness-like reactions consisting of erythema multiforme or maculopapular pruritic rash or urticaria accompanied by arthritis, arthralgia, and fever have been reported rarely in patients receiving cefaclor. These reactions usually have occurred in pediatric patients younger than 6 years of age, most often with second or subsequent courses of the drug. While serum sickness-like reactions have been reported rarely in patients receiving other cephalosporins (i.e., cefprozil, cephalexin) or other b-lactam antibiotics (i.e., amoxicillin, loracarbef), these reactions been reported more frequently with cefaclor than with any other anti-infective agent. (See Cautions: Adverse Effects in Cefaclor 8:12.06.08.)
Hematologic Effects
Positive direct and indirect antiglobulin (Coombs’) test results have been reported in 3% or more of patients receiving a cephalosporin. (See Laboratory Test Interferences: Immunohematology Tests.) The mechanism of this reaction is usually nonimmunologic in nature; a cephalosporin-globulin complex coats the erythrocytes and reacts nonspecifically with Coombs’ serum. Nonimmunologic positive Coombs’ test results are most likely to occur in patients who have received large doses of a cephalosporin or who have impaired renal function or hypoalbuminemia.
Other adverse hematologic effects of cephalosporins include rare, mild and transient neutropenia, thrombocythemia or thrombocytopenia, leukocytosis, granulocytosis, monocytosis, lymphocytopenia, basophilia, and reversible leukopenia.
Transient lymphocytosis has been reported occasionally in infants and children receiving cefaclor. Rarely, decreased hemoglobin and/or hematocrit, anemia, and agranulocytosis have been reported with some cephalosporins.
Aplastic anemia, pancytopenia, hemolytic anemia, and epistaxis or hemorrhage also have been reported with cephalosporin therapy. Immune-mediated hemolytic anemia with extravascular hemolysis, including some fatalities, have been reported in patients receiving cefotaxime, ceftizoxime, ceftriaxone, or cefotetan (a cephamycin).
Prolonged prothrombin time (PT), prolonged activated partial thromboplastin time (APTT), and/or hypoprothrombinemia (with or without bleeding) have been reported rarely with cefaclor, cefixime, cefoperazone, cefotaxime, cefotetan, cefoxitin, ceftizoxime, ceftriaxone, and cefuroxime.
Although the true incidence of hypoprothrombinemia and bleeding complications during therapy with these drugs has not been established, these effects have been reported most frequently with drugs that contain an NMTT side chain (e.g., cefamandole [no longer commercially available in the US], cefoperazone) and have usually occurred in geriatric, debilitated, or other patients with vitamin K deficiency or in patients with severe renal failure or following radical GI surgery. The mechanism(s) of the hypoprothrombinemic effect of these drugs has not been clearly established.
While hypoprothrombinemia with some of the drugs may result in part from a reduction in vitamin K-producing bacteria in the GI tract, there is evidence that a direct effect on hepatic synthesis of prothrombin or prothrombin precursors is involved. The NMTT side chain apparently interferes with vitamin K metabolism and regeneration and inhibits γ-carboxylation of glutamic acid, the vitamin K-dependent step in the hepatic synthesis of prothrombin.
Hypoprothrombinemia has usually been reversed by administration of vitamin K. Prophylactic administration of vitamin K may be indicated in geriatric, debilitated, or other patients with vitamin K deficiency or those at risk of vitamin K deficiency during cefoperazone therapy. Vitamin K should also be administered when indicated to treat hypoprothrombinemia associated with use of other cephalosporins.
Renal and Genitourinary Effects
Renal effects that have occurred occasionally with administration of a cephalosporin include transient increases in BUN and serum creatinine concentrations, renal dysfunction, and toxic nephropathy.
Nephrotoxicity has been reported rarely with cephalexin and cefazolin and acute renal failure has been reported with cefazolin and cefixime. Reversible interstitial nephritis has occurred rarely during cefaclor therapy, and purpuric nephritis has been reported with oral cefpodoxime proxetil therapy in postmarketing experience outside the US. Renal toxicity is most likely to occur in patients older than 50 years of age, patients with prior renal impairment, or patients who are receiving other nephrotoxic drugs. (See Drug Interactions: Nephrotoxic Drugs.)
All cephalosporins should be administered with caution and in reduced dosage in the presence of markedly impaired renal function. In patients with suspected renal impairment, careful clinical observation and renal function tests should be performed prior to and during cephalosporin therapy.
Genitourinary effects reported with cephalosporin therapy include vaginitis, vaginal candidiasis, genital pruritus, and menstrual irregularities.
Hepatic Effects
Transient increases in serum AST (SGOT), ALT (SGPT), γ-glutamyl transferase (?-glutamyl transpeptidase, GGT, GGTP), and alkaline phosphatase concentrations have occurred occasionally with cephalosporin therapy. Increased serum concentrations of bilirubin and/or LDH have been reported with many cephalosporins; decreased serum albumin and/or total protein also have been reported.
Hepatic dysfunction, including cholestasis, also has been reported with cephalosporin therapy. Hepatitis and/or jaundice have been reported with cefazolin, cefixime, and ceftazidime. Isolated instances of hepatomegaly have been reported with cephradine.
These hepatic effects are generally mild and disappear when cephalosporin therapy is discontinued. Acute liver injury has been reported with oral cefpodoxime proxetil therapy in postmarketing experience outside the US.
GI Effects
The most frequent adverse reactions to orally administered cephalosporins are nausea, vomiting, and diarrhea.
These effects are usually mild and transient, but rarely may be severe enough to require discontinuance of the drug. Other adverse GI effects which have occurred with some of the oral cephalosporins include abdominal pain, tenesmus, epigastric pain/dyspepsia, decreased appetite/anorexia, glossitis, flatulence, candidiasis (e.g., oral thrush), taste alteration, decreased salivation, and heartburn. Adverse GI effects can also occur with IM or IV cephalosporins.
Rarely, Clostridium difficile-associated diarrhea and colitis (also known as antibiotic-associated pseudomembranous colitis) has occurred during or following discontinuance of cephalosporins, including following single doses of certain cephalosporins; fatalities have been reported rarely. Mild cases of C. difficile-associated diarrhea and colitis may respond to discontinuance of the cephalosporin alone, but diagnosis and management of moderate to severe cases should include appropriate bacteriologic studies and treatment with fluid, electrolyte, and protein supplementation as indicated.
Geriatric patients may be particularly susceptible to fluid losses and should be treated aggressively. Antiperistaltic agents (e.g., opiates, diphenoxylate with atropine) may prolong and/or worsen the condition and should be avoided if pseudomembranous colitis is suspected. If colitis is moderate to severe or is not relieved by discontinuance of the cephalosporin, appropriate anti-infective therapy (e.g., oral metronidazole or vancomycin) should be administered. Isolation of the patient may be advisable. Other causes of colitis should also be considered.
Local Effects
Local reactions are quite common following IM or IV administration of some parenteral cephalosporins; phlebitis and thrombophlebitis occasionally occur with IV administration of the drugs. Although reports are conflicting and results of many studies inconclusive, there appears to be little difference in the overall incidence of mild phlebitis and thrombophlebitis among the currently available IV cephalosporins.
Nervous System Effects
Nervous system effects that have occurred following oral, IM, or IV administration of cephalosporins include dizziness, headache, malaise, fatigue, nightmares, and vertigo. Hyperactivity, nervousness or anxiety, agitation, hallucinations, insomnia, somnolence, weakness, hot flushes, alteration in color perception, confusion, and hypertonia also have been reported during therapy with some cephalosporins, although a causal relationship has not necessarily been established.
Mild to moderate hearing loss has been reported in a few pediatric patients receiving cefuroxime sodium for the treatment of meningitis. In patients with renal insufficiency, elevated concentrations of ceftazidime reportedly may lead to seizures, encephalopathy, asterixis, and neuromuscular excitability. Seizures also have been reported with cefixime and cefuroxime.
Rarely, toxic paranoid reactions have occurred in patients with renal impairment receiving oral cephalexin. Intrathecal administration of cephalosporins, particularly in large doses, has resulted in CNS toxicity evidenced by hallucinations, nystagmus, and seizures. (See Cautions: Precautions and Contraidications.)
Other Adverse Effects
Other adverse effects reported with cephalosporin therapy include chest pain, pleural effusion, pulmonary infiltrate, dyspnea or respiratory distress, cough, and rhinitis. Increased or decreased serum glucose concentration also has been reported.
Tightness in the chest and paresthesia have been reported following oral, IM, or IV administration of cephradine. Fungal skin infection, exacerbation of acne, and ocular itching have been reported rarely in patients receiving oral cefpodoxime proxetil therapy.
An anamnestic photosensitivity (photo recall)-like dermatitis, characterized by a pruritic, erythematous, maculopapular eruption distributed in the area of a recent sunburn on the upper abdomen and chest, has been reported in at least one patient receiving concomitant IV therapy with cefazolin and gentamicin sulfate. Whether this phenomenon was caused by one or both drugs has not been determined; however, the reaction resolved within 48 hours following discontinuance of both drugs.
Precautions and Contraindications
Prior to initiation of cephalosporin therapy, careful inquiry should be made concerning previous hypersensitivity reactions to cephalosporins, penicillins, and other drugs. Cephalosporins are contraindicated in patients with a history of allergic reactions to cephalosporin antibiotics. There is clinical and laboratory evidence of partial cross-sensitivity among bicyclic b-lactam antibiotics including penicillins, cephalosporins, cephamycins, and carbapenems.
There appears to be little cross-sensitivity between bicyclic b-lactam antibiotics and monobactams (e.g., aztreonam). Although the true incidence of cross-sensitivity among the b-lactam antibiotics has not been definitely established, it has been clearly documented and may occur in up to 10-15% of patients with a history of penicillin hypersensitivity. Some patients have had severe reactions, including anaphylaxis, to both penicillins and cephalosporins. Cephalosporins should be used with caution in individuals hypersensitive to penicillins.
Some clinicians suggest that cephalosporins should be avoided in patients who have had an immediate-type (anaphylactic) hypersensitivity reaction to penicillins and should be administered with caution to patients who have had a delayed-type (e.g., rash, fever, eosinophilia) reaction to penicillins or other drugs. If an allergic reaction occurs during cephalosporin therapy, the drug should be discontinued and the patient treated with appropriate therapy (e.g., epinephrine, corticosteroids, and maintenance of an adequate airway and oxygen) as indicated.
Prolonged use of a cephalosporin may result in the overgrowth of nonsusceptible organisms, especially Enterobacter, Pseudomonas, enterococci, or Candida. If superinfection occurs, appropriate therapy should be instituted.
Cephalosporins should be used with caution in patients with a history of GI disease, particularly colitis. Because C. difficile-associated diarrhea and colitis has been reported with the use of cephalosporins, it should be considered in the differential diagnosis of patients who develop diarrhea during or following therapy with the drugs.
Seizures have been reported with several cephalosporins (e.g., ceftazidime, cefuroxime), particularly in patients with renal impairment in whom dosage of the drug was not reduced. (See Cautions: Nervous System Effects.)
If seizures occur during cephalosporin therapy, the drug should be discontinued and anticonvulsant therapy initiated as clinically indicated. Because some cephalosporins have been associated with a decrease in prothrombin activity, some manufacturers state that prothrombin time (PT) should be monitored when these drugs are used in patients with renal or hepatic impairment, in patients with poor nutritional status, in patients receiving a protracted course of anti-infective therapy, or in those previously stabilized on anticoagulant therapy.
Vitamin K should be administered if indicated.
Pregnancy, Fertitlity and Lactation
Although there have been no reports of adverse effects to the fetus to date, safe use of cephalosporins during pregnancy has not been definitely established. The drugs should be used during pregnancy only when clearly needed.
Adverse testicular effects (e.g., reduced testicular weight, seminiferous tubule degeneration, delayed maturity of germinal epithelium, reduced germinal cell population, vacuolation of Sertoli cell cytoplasm) have been reported in prepubertal rats receiving b-lactam anti-infective agents that contain an NMTT side chain (e.g., cefamandole [no longer commercially available in the US], cefoperazone, cefotetan); impaired fertility also has been reported, particularly with high dosages.
The relevance of these findings to humans is not known. Because cephalosporins are distributed into milk, the drugs should be used with caution in nursing women.
Drug Interactions
Alcohol
Disulfiram-like reactions have occurred when alcohol was ingested within 48-72 hours after administration of b-lactam antibiotics that contain an N-methylthiotetrazole (NMTT) side chain (e.g., cefamandole [no longer commercially available in the US], cefoperazone, cefotetan).
The reactions appear to result from accumulation of acetaldehyde and do not occur if alcohol is ingested prior to the first dose of the antibiotic. Ingestion of alcohol during and for 24-72 hours after administration of cefoperazone or cefotetan should be avoided.
Probenecid
Concomitant administration of oral probenecid competitively inhibits tubular secretion resulting in higher and more prolonged serum concentrations of most cephalosporins.
Nephrotoxic Drugs
Concurrent use of nephrotoxic agents such as aminoglycosides, colistin, polymyxin B, or vancomycin may increase the risk of nephrotoxicity with some cephalosporins and probably should be avoided, if possible.
Other Anti-infective Agents
In vitro studies indicate that the antibacterial activity of cephalosporins may be additive or synergistic with aminoglycosides or penicillins against some organisms. Although some in vitro studies showed additive or synergistic antibacterial activity between chloramphenicol and a cephalosporin, there is more recent in vitro evidence of antagonism between cephalosporins (e.g., cefoperazone, cefotaxime, ceftazidime, ceftriaxone) and chloramphenicol against a variety of gram-negative and -positive bacteria, particularly when chloramphenicol was added to the medium before the b-lactam. In addition, at least one case of in vivo antagonism has been reported in an infant with Salmonella enteritidis meningitis. Therefore, it is recommended that combined therapy with chloramphenicol and a cephalosporin be avoided, particularly when bactericidal activity is considered important.
Laboratory Test Interferences
Immunohematology Tests Positive direct and indirect antiglobulin (Coombs’) test results have been reported in 3% or more of patients receiving a cephalosporin. (See Cautions: Hematologic Effects.) This reaction may interfere with hematologic studies or transfusion cross-matching procedures. In addition, false-positive Coombs’ test results may occur in neonates whose mother received a cephalosporin prior to delivery.
Tests for Urinary Glucose
Cephalosporins can cause false-positive results in urine glucose determinations using cupric sulfate solution (Benedict’s reagent, Clinitest®); glucose oxidase tests (Clinistix®) are unaffected by the drugs.
Tests for Creatinine
Some cephalosporins, in high concentrations, may cause falsely elevated serum or urine creatinine values when the Jaffe reaction is used. In one in vitro study, high concentrations of ceftriaxone (50 mcg/mL or greater) caused falsely elevated serum creatinine values when a manual method was used; however, other studies indicate that the drug does not interfere with automated methods for determining serum or urinary creatinine concentrations.
Mechanism of Action
Cephalosporins are usually bactericidal in action. The antibacterial activity of the cephalosporins, like penicillins, carbacephems, and cephamycins, results from inhibition of mucopeptide synthesis in the bacterial cell wall. Although the exact mechanisms of action of cephalosporins have not been fully elucidated, b-lactam antibiotics bind to several enzymes in the bacterial cytoplasmic membrane (e.g., carboxypeptidases, endopeptidases, transpeptidases) that are involved in cell-wall synthesis and cell division. It has been hypothesized that b-lactam antibiotics act as substrate analogs of acyl-D-alanyl-D-alanine, the usual substrate for these enzymes.
This interferes with cell-wall synthesis and results in the formation of defective cell walls and osmotically unstable spheroplasts. Cell death following exposure to b-lactam antibiotics usually results from lysis, which appears to be mediated by bacterial autolysins such as peptidoglycan hydrolases. The target enzymes of b-lactam antibiotics have been classified as penicillin-binding proteins (PBPs) and appear to vary substantially among bacterial species.
The affinities of various b-lactam antibiotics for different PBPs appear to explain the differences in morphology that occur in susceptible organisms following exposure to different b-lactam antibiotics and may also explain differences in the spectrum of activity of b-lactam antibiotics that are not caused by the presence or absence of b-lactamases. Spectrum In general, cephalosporins are active in vitro against many gram-positive aerobic bacteria, some gram-negative aerobic bacteria, and some anaerobic bacteria; however, there are substantial differences among the cephalosporins in spectra of activity as well as levels of activity against susceptible bacteria. Cephalosporins are inactive against fungi and viruses.
Classification of Cephalosporins and Closely Related β-Lactam Antibiotics
Based on Spectra of Activity Currently available cephalosporins are generally divided into 4 groups based on their spectra of activity: first, second, third, and fourth generation cephalosporins. Closely related b-lactam antibiotics (e.g., cephamycins) also may be classified in these groups because of their similar spectra of activity.
FIRST GENERATION CEPHALOSPORINS
First generation cephalosporins usually are active in vitro against gram-positive cocci including penicillinase-producing and nonpenicillinase-producing Staphylococcus aureus and S. epidermidis; Streptococcus pyogenes (group A b-hemolytic streptococci); S. agalactiae (group B streptococci); and S. pneumoniae. First generation cephalosporins have limited activity against gram-negative bacteria, although some strains of Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, and Shigella may be inhibited in vitro by the drugs. First generation cephalosporins are inactive against enterococci, including Enterococcus faecalis (formerly S. faecalis), oxacillin-resistant staphylococci (formerly known as methicillin-resistant staphylococci), Bacteroides fragilis, Citrobacter,Enterobacter, Listeria monocytogenes, Proteus other than P. mirabilis, Providencia, Pseudomonas, and Serratia.
Susceptible strains of S. aureus, S. pneumoniae, S. pyogenes, or S. agalactiae usually are inhibited in vitro by cefazolin concentrations of 0.1-1 mcg/mL or cephradine or cephalexin concentrations of 0.1-12 mcg/mL. S. epidermidis may be inhibited in vitro by cefazolin concentrations of 0.1-12. mcg/mL, although some strains require concentrations greater than 32 mcg/mL for in vitro inhibition. Susceptible strains of E. coli, K. pneumoniae, or P. mirabilis generally are inhibited in vitro by cefazolin or cephradine concentrations of 0.8-12.5 mcg/mL.
SECOND GENERATION CEPHALOSPORINS
Second generation cephalosporins usually are active in vitro against bacteria susceptible to first generation cephalosporins. In addition, the second generation drugs are active in vitro against most strains of Haemophilus influenzae (including ampicillin-resistant strains).
Although the specific spectra of activity differ, second generation cephalosporins generally are more active in vitro against gram-negative bacteria than first generation cephalosporins. However, cefaclor is less active than other second generation cephalosporins against gram-negative bacteria. The second generation drugs may be active in vitro against some strains of Acinetobacter, Citrobacter, Enterobacter, E. coli, Klebsiella, Neisseria, Proteus, Providencia, and Serratia that are resistant to the first generation drugs.
Cefotetan and cefoxitin also have some activity in vitro against B. fragilis. Second generation cephalosporins are inactive against enterococci (e.g., E. faecalis), oxacillin-resistant staphylococci, and Pseudomonas. For more complete information on the spectra of activity of cefotetan, cefoxitin, and cefuroxime, see the individual monographs in 8:12.06.08 or 8:12.07..
THIRD GENERATION CEPHALOSPORINS
Third generation cephalosporins usually are less active in vitro against susceptible staphylococci than first generation cephalosporins; however, the third generation drugs have an expanded spectrum of activity against gram-negative bacteria compared with the first and second generation drugs.
Third generation cephalosporins generally are active in vitro against gram-negative bacteria susceptible to the first and second generation drugs, and most also are active in vitro against Citrobacter, Enterobacter, E. coli, Klebsiella, Neisseria, Proteus, Morganella, Providencia, and Serratia that may be resistant to first and second generation cephalosporins. Some parenteral third generation drugs have activity in vitro against B. fragilis and Pseudomonas. Cefdinir, cefixime, and cefpodoxime are inactive against most strains of Enterobacter and Pseudomonas and have limited in vitro activity against anaerobic bacteria; cefixime also is inactive against most staphylococci. Cefditoren has increased activity, similar to first generation cephalosporins, against gram-positive bacteria, unlike other third generation cephalosporins. Third generation cephalosporins are inactive against oxacillin-resistant staphylococci and generally are inactive against enterococci (e.g., E. faecalis) and L. monocytogenes. For more complete information on the spectra of activity of the third generation cephalosporins, see the individual monographs in 8:12.06.12.
FOURTH GENERATION CEPHALOSPORINS
Fourth generation cephalosporins, like third generation cephalosporins, have an expanded spectrum of activity against gram-negative bacteria compared with the first and second generation drugs.
However, fourth generation cephalosporins are active in vitro against some gram-negative bacteria, including Pseudomonas aeruginosa and certain Enterobacteriaceae, that generally are resistant to third generation cephalosporins.
In addition, fourth generation cephalosporins may be more active against gram-positive bacteria than some third generation drugs (e.g., ceftazidime). Cefepime has a spectrum of activity against aerobic gram-positive and gram-negative bacteria that is similar to that of cefotaxime, ceftriaxone, and ceftizoxime and has activity against Ps. aeruginosa that appears to approach that of ceftazidime. However, cefepime is more active than third generation cephalosporins against Enterobacteriaceae that produce inducible b-lactamases. The extended spectrum of activity of cefepime is related to the fact that the drug penetrates the outer membrane of gram-negative bacteria more rapidly than most other cephalosporins and the fact that the drug is more resistant to inactivation by chromosomally and plasmid-mediated b-lactamases than most other cephalosporins. In addition, inducible b-lactamases have a low affinity for cefepime and the drug is hydrolyzed by these enzymes at a slower rate than third generation cephalosporins such as ceftazidime. Like other cephalosporins, cefepime is inactive against oxacillin-resistant staphylococci, enterococci, and L. monocytogenes.
In Vitro Susceptibility Testing
Many factors such as inoculum size, pH, and test media can influence results of cephalosporin in vitro susceptibility tests. When the disk-diffusion procedure is used for in vitro susceptibility testing, the National Committee for Clinical Laboratory Standards (NCCLS) states that a cephalosporin class disk containing 30 mcg of cephalothin may be used to test susceptibility to most first generation cephalosporins (i.e., cefadroxil, cephalexin, cephradine) and to test susceptibility to cefaclor, a second generation cephalosporin. Although cefazolin is a first generation cephalosporin, NCCLS recommends that a separate disk containing 30 mcg of cefazolin be used to test susceptibility to the drug.
Because there are differences in the spectra of activity of cefdinir, cefditoren, cefepime, cefixime, cefoperazone, cefotaxime, cefotetan, cefoxitin, cefpodoxime, cefprozil, ceftazidime, ceftibuten, ceftizoxime, ceftriaxone, and cefuroxime, NCCLS recommends that these drugs be tested individually with separate drug-specific disks to determine in vitro susceptibility.
Resistance Bacterial resistance to cephalosporins may be natural or acquired and may result from one or a combination of factors. Some bacteria are not affected by concentrations of a cephalosporin that are lethal to other bacteria because their cell surfaces are not permeable to the drug or because their metabolic pathways are not inhibited by the drug. A major mechanism of bacterial resistance to cephalosporins is the production of b-lactamases which inactivate the drugs by hydrolyzing the b-lactam ring.
However, absence or presence of a b-lactamase does not entirely dictate susceptibility or resistance to a cephalosporin.
Bacterial resistance usually results both from the production of a b-lactamase and the presence of permeability barriers to the drug.
The b-lactamases produced by different bacterial species differ in physical, chemical, and functional properties. Staphylococcal b-lactamases are usually inducible, extracellular penicillinases. A variety of b-lactamases are produced by gram-negative bacteria; some are chromosome-mediated inducible cephalosporinases and a few are determined by plasmid-mediated resistance factors.
Pharmacokinetics
Absorption
Cefazolin sodium, cefepime hydrochloride, cefoperazone sodium, cefotaxime sodium, ceftazidime, ceftizoxime sodium, ceftriaxone sodium, and cefuroxime sodium are not appreciably absorbed from the GI tract and must be given parenterally.
Cefaclor, cefadroxil, cefprozil, ceftibuten dihydrate, cephalexin, cephalexin hydrochloride, and cephradine are well absorbed from the GI tract. Following oral administration, bioavailability of cefdinir averages 16-25%.
Approximately 30-50% of an oral dose of cefixime and 50% of an oral dose of cefpodoxime proxetil is absorbed from the GI tract. Cefpodoxime proxetil is a prodrug and is inactive until hydrolyzed in vivo to cefpodoxime by nonspecific esterases in the intestinal lumen. Cefditoren pivoxil also is a prodrug and is inactive until hydrolyzed in vivo by esterases to cefditoren.
Bioavailability of oral cefuroxime axetil averages 37-52%; the drug is inactive until hydrolyzed in vivo to cefuroxime by nonspecific esterases in the intestinal mucosa and blood. Administration with food does not affect the rate of absorption or peak serum concentrations of cefadroxil. While the rate of absorption of cefixime or cefprozil may be decreased by the presence of food in the GI tract, the extent of absorption and peak plasma concentrations of the drug generally are not affected. Compared with administration in the fasting state, administration of cefdinir with a high-fat meal decreases the rate and extent of absorption of the drug. Administration of cefditoren pivoxil with a moderate- or high-fat meal increases the rate and extent of absorption, compared with administration in the fasting state. The effect of food on oral bioavailability of cefaclor, cefpodoxime proxetil, ceftibuten, and cefuroxime axetil varies depending on the formulation of the drugs administered.
Distribution and Elimination
Following absorption, most cephalosporins are widely distributed to tissues and fluids, including pleural fluid, synovial fluid, and bone. Following oral administration, cefdinir, cefixime, cefpodoxime, ceftibuten, cefprozil, and cefuroxime are distributed into middle ear fluid, tonsils, sinus tissue, and bronchial mucosa. Following oral administration, cefditoren has been shown to distribute into tonsils and skin blister fluid.
Although the total quantity of some cephalosporins distributed into bile is low, therapeutic concentrations of some of the drugs (e.g., cefazolin, cefepime, cefixime, cefoperazone) generally are obtained if biliary obstruction is not present. Only low concentrations of first or second generation cephalosporins diffuse into CSF following oral, IM, or IV administration even when meninges are inflamed; however, therapeutic concentrations of cefotaxime, ceftazidime, ceftizoxime, ceftriaxone, or cefuroxime generally are attained in CSF following IM or IV administration, especially if meninges are inflamed. Cefepime also is distributed into CSF following parenteral administration.
Cephalosporins readily cross the placenta, and fetal serum concentrations may be 10% or more of maternal serum concentrations. Cephalosporins are distributed in low concentrations into milk. The serum half-lives of cephalosporins in adults with normal renal function and approximate degrees of protein binding have been reported as follows: Drug Serum Half-life (in hours) % Bound to Serum Proteins FIRST GENERATION Cefadroxil 1.1-2 20 Cefazolin 1.2-2. 74-86 Cephalexin 0.5-1. 6-15 Cephradine 0.7-2 6-20 SECOND GENERATION Cefaclor 0.5-1 25 Cefprozil 1-1. 35-45 Cefuroxime 1-2 33-50 THIRD GENERATION Cefdinir 1.7-1. 60-70 Cefditoren 1.4-1. 88 Cefixime 2.4-4 65-70 Cefoperazone 1.6-2. 82-93 Cefotaxime 0.9-1. 13-38 Cefpodoxime 2.1-2. 22-33 Ceftazidime 1.4-2 5-24 Ceftibuten 2-2. 65 Ceftizoxime 1.4-1. 28-31 Ceftriaxone 5.4-10. 58-96 FOURTH GENERATION Cefepime 2-2. 20 Serum cephalosporin concentrations may be higher and the serum half-lives prolonged in patients with impaired renal function. Cefaclor, cefadroxil, cefazolin, cefdinir, cefditoren, cefixime, cefpodoxime, cefprozil, ceftazidime, ceftizoxime, cefuroxime, cephalexin, and cephradine are not appreciably metabolized. Cefuroxime axetil, cefditoren pivoxil, and cefpodoxime proxetil are rapidly hydrolyzed to their respective microbiologically active forms, cefuroxime, cefditoren, and cefpodoxime, by nonspecific esterases in the intestinal mucosa and/or blood following oral administration.
The axetil moiety of cefuroxime is metabolized to acetaldehyde and acetic acid; hydrolysis of cefditoren pivoxil results in the formation of pivalate, which is absorbed and excreted as pivaloylcarnitine in urine.
Cefepime is partially metabolized in vivo. Cefotaxime is partially metabolized (presumably in the liver, kidneys, and other tissues) to desacetyl metabolites which also have antibacterial activity, although less than that of the parent compounds. Ceftriaxone is metabolized to a small extent to microbiologically inactive metabolites in the intestines after biliary excretion.
Cephalosporins and their metabolites are rapidly excreted by the kidneys. Cefaclor, cefadroxil, cefazolin, cefditoren, cefixime, cefoperazone, cefotaxime, cefpodoxime, cefprozil, ceftizoxime, cefuroxime, cephalexin, and cephradine are excreted by both glomerular filtration and tubular secretion; cefepime, ceftazidime, and ceftriaxone are excreted principally by glomerular filtration. The same system of anion transport is responsible for the tubular secretion of cephalosporins as for other b-lactam antibiotics and probenecid. Oral probenecid administered shortly before or with most cephalosporins usually slows the rate of excretion of the antibiotic and produces higher and more prolonged serum concentrations, especially with those cephalosporins excreted principally by tubular secretion.
Most cephalosporins are removed by hemodialysis or peritoneal dialysis.
Chemistry and Stability
Chemistry
Cephalosporins are semisynthetic antibiotic derivatives of cephalosporin C, a substance produced by the fungus Cephalosporium acremonium. The drugs are b-lactam antibiotics structurally and pharmacologically related to penicillins, carbacephems (e.g., loracarbef), and cephamycins (e.g., cefotetan, cefoxitin).
All commercially available cephalosporins contain the 7-aminocephalosporanic acid (7-ACA) nucleus, which is composed of a b-lactam ring fused with a 6-membered dihydrothiazine ring instead of the 5-membered thiazolidine ring of penicillins. Cleavage at any point in the b-lactam ring system of cephalosporins results in complete loss of antibacterial activity. Addition of various groups at R1 (position 7) and R2 (position 3) of the cephalosporin nucleus results in derivatives with differences in spectra of activity, stability against hydrolysis by b-lactamases, protein binding, GI absorption, or susceptibility to desacetylation.
Many commercially available oral cephalosporins (e.g., cefdinir, cefditoren pivoxil, cefixime, cefpodoxime proxetil, ceftibuten) and parenteral cephalosporins (e.g., cefepime, cefotaxime, ceftazidime, ceftizoxime, ceftriaxone) contain an aminothiazolyl side chain at position 7 of the cephalosporin nucleus.
The aminothiazolyl side chain enhances antibacterial activity, particularly against Enterobacteriaceae, and generally results in enhanced stability against b-lactamases.
Some commercially available cephalosporins (e.g., cefoperazone) and structurally related cephamycins (e.g., cefotetan) contain anN-methylthiotetrazole (NMTT) side chain at position 6 of the cephalosporin nucleus. The NMTT side chain enhances antibacterial activity, helps to prevent metabolism of the drugs, and also may be associated with certain adverse effects (e.g., hypoprothrombinemia, disulfiram-like reactions).
Stability
In solution, most cephalosporins are stable for only short periods of time unless frozen. Cephalosporins are potentially physically and/or chemically incompatible with some drugs including aminoglycosides, but the compatibility depends on the specific drug and several other factors (e.g., concentration of the drugs, specific diluents used, resulting pH, temperature). Specialized references should be consulted for specific compatibility information. For specific dosages and additional information on chemistry and stability, spectrum, resistance, pharmacokinetics, uses, cautions, drug interactions, and laboratory test interferences of the cephalosporins, see the individual monographs in 8:12.06.