Ciprofloxacin generally is well tolerated, and adverse effects of the drug are similar to those reported with other quinolone anti-infectives (e.g., norfloxacin, ofloxacin). Adverse effects have been reported in 5-14% of patients receiving ciprofloxacin, and have been severe enough to require discontinuance in 2-3.5% of patients. The most frequent adverse effects of the drug involve the GI tract or CNS; those requiring discontinuance of the drug also principally involve these organ systems.
Limited data currently are available regarding adverse effects of ciprofloxacin in individuals receiving anthrax postexposure prophylaxis regimens. In response to a questionnaire given to 490 such individuals in Florida on approximately day 7 or 14 of anti-infective prophylaxis, 19% sought medical attention for any anti-infective related adverse effect or reported one or more of the following: pruritus, breathing problems, or swelling of the face, neck, or throat.
Although the percentage of patients in this subgroup who received ciprofloxacin versus other anti-infectives was not reported, 86% of all patients (i.e., those who did or did not answer the questionnaire) received ciprofloxacin and 80% continued to receive prophylaxis beyond 14 days. In an epidemiologic evaluation in 8424 postal workers who were offered 60 days of prophylaxis for anthrax and given a questionnaire in New Jersey, New York City, and the District of Columbia on days 7-10 of anti-infective prophylaxis, 5819 completed or were administered the questionnaire, of whom 3863 had initiated prophylaxis (3428 with ciprofloxacin).
Of the ciprofloxacin-treated individuals, 19% reported severe nausea, vomiting, diarrhea, and/or abdominal pain; 14% reported fainting, light-headedness, and/or dizziness; 7% reported heartburn or acid reflux; 6% reported rash, urticaria, and/or pruritus; and 8% discontinued therapy with the drug (3% for adverse effects, 1% for fear of developing an adverse effect, and 1% because they were confused about the need). Only 2% of those on any anti-infective sought medical attention for possible manifestations of anaphylaxis, none of whom required hospitalization.
GI Effects
Nausea,diarrhea, vomiting, and abdominal pain/discomfort have been reported in 2-10% of patients receiving ciprofloxacin. These effects generally are mild and transient and occur most frequently in geriatric patients and/or when high dosage is used. Anorexia, dyspepsia, flatulence, GI erosion and bleeding, dysphagia, bad taste,intestinal perforation, painful oral mucosa, and oral candidiasis have been reported in less than 1% of patients receiving the drug.
Effects on Fecal Flora
Ciprofloxacin exerts a selective effect on normal bowel flora. Although fluoroquinolones, including ciprofloxacin, are relatively inactive against Clostridium difficile in vitro, C. difficile-associated diarrhea and colitis (also known as antibiotic-associated pseudomembranous colitis) has been reported in only 1% or less of patients receiving the drugs. In addition, C. difficile-associated diarrhea and colitis produced by other anti-infectives (e.g., ceftriaxone) has resolved in several patients following discontinuance of these other anti-infectives and initiation of ciprofloxacin.
The reason for this relative lack of association between ciprofloxacin and colitis has not been elucidated but may be related to achievement of fecal concentrations of the drug that substantially exceed the MIC of C. difficile and/or the selective effect of the drug on normal GI flora. It should be noted, however, that initiation of ciprofloxacin in these patients may not have been responsible for resolution of the colitis but may have only passively allowed such resolution to occur following discontinuance of the offending anti-infective.
The possibility that diarrhea developing in any ciprofloxacin-treated patient may be secondary to C. difficile-associated colitis should be considered. C. difficile-associated diarrhea and colitis can range in severity from mild to life-threatening. Mild cases of colitis may respond to discontinuance of ciprofloxacin alone, but diagnosis and management of moderate to severe cases should include appropriate bacteriologic studies and treatment with fluid, electrolyte, and protein supplementation as indicated. If colitis is moderate to severe or is not relieved by discontinuance of ciprofloxacin, appropriate anti-infective therapy (e.g., oral metronidazole or vancomycin) should be administered. Isolation of the patient may be advisable. Other causes of colitis also should be considered.
Total bacterial counts of normal anaerobic fecal flora generally are unaffected during or following ciprofloxacin therapy. However, total bacterial counts of normal aerobic fecal flora are decreased within 2-5 days following initiation of therapy with the drug and generally return to pretreatment levels within 1-4 weeks after the drug is discontinued. Ciprofloxacin therapy generally markedly reduces or completely eradicates normal fecal Enterobacteriaceae; the drug reduces fecal aerobic gram-positive bacteria to a lesser extent. Ciprofloxacin therapy does not appear to affect total bacterial counts of normal salivary flora, including streptococci, staphylococci, and anaerobic bacteria.
Nervous System Effects
Headache and restlessness have been reported in about 1-2% of patients receiving ciprofloxacin. Dizziness, lightheadedness, insomnia, nightmares, hallucinations, manic reaction, toxic psychosis, irritability, tremor, ataxia, seizures, lethargy, drowsiness, vertigo, anxiety,nervousness, confusion, weakness, malaise, phobia, depersonalization, depression, suicidal thoughts or acts, and paresthesia, and increased intracranial pressure have been reported in less than 1% of patients. Some of these reactions may occur following the first dose. If seizures or other severe CNS reactions occur during ciprofloxacin therapy, the drug should be discontinued and appropriate measures instituted. (See Precautions and Contraindications.)
Some adverse nervous system effects of ciprofloxacin may be related to the fact that the drug, like other fluoroquinolones, is a ?-aminobutyric acid (GABA) inhibitor. In addition, it has been suggested that some CNS stimulant effects reported in patients receiving the drug may have resulted from ciprofloxacin-induced alterations in caffeine pharmacokinetics. (See Drug Interactions: Xanthine Derivatives.)
Dermatologic and Sensitivity Reactions
Mild, transient rash has been reported in 1-4% and eosinophilia, pruritus, urticaria, cutaneous candidiasis, hyperpigmentation, erythema nodosum, angioedema, and edema of the face, neck, lips, conjunctivae, or hands have been reported in less than 1% of patients. Flushing, fever, chills, and photosensitivity also have been reported in less than 1% of patients receiving the drug. Severe hypersensitivity reactions characterized by rash, fever, eosinophilia, jaundice, and hepatic necrosis and that were fatal have been reported rarely in patients receiving ciprofloxacin and other drugs concomitantly.
Toxic epidermal necrolysis also has been reported rarely in patients receiving ciprofloxacin. The possibility that these reactions were related to ciprofloxacin therapy could not be excluded. Therefore, the manufacturer recommends that ciprofloxacin be discontinued at the first sign of rash or any other sign of hypersensitivity. In addition, serious and occasionally fatal hypersensitivity (anaphylactic and anaphylactoid) reactions have occurred, some with the initial dose, in patients receiving quinolone therapy. Some such reactions were accompanied by cardiovascular collapse, loss of consciousness, paresthesia, pharyngeal or facial edema, dyspnea, urticaria, and/or pruritus; there was a history of hypersensitivity in only a few of these cases.
Limited evidence suggests that the frequency of severe hypersensitivity reactions may be higher in patients with acquired immunodeficiency syndrome (AIDS) than in other patients; the exact mechanism(s) of this increased risk of ciprofloxacin toxicity has not been determined. If a severe hypersensitivity reaction occurs during ciprofloxacin therapy, the drug should be discontinued and the patient given appropriate therapy (e.g., epinephrine, corticosteroids, maintenance of an adequate airway, oxygen, maintenance of blood pressure) as indicated.
Genitourinary Effects
Increased serum creatinine and BUN concentrations have occurred in about 1% of patients receiving ciprofloxacin. Interstitial nephritis, nephritis, renal failure, dysuria, polyuria, urinary retention, albuminuria, urethral bleeding, vaginitis, and acidosis have been reported in less than 1% of patients receiving the drug. In at least one patient, acute renal failure associated with interstitial nephritis occurred within about 2 weeks after initiating ciprofloxacin and appeared to be a hypersensitivity reaction to ciprofloxacin; renal biopsy showed marked interstitial edema, with extensive lymphocytic infiltrations and occasional eosinophils. Crystalluria, cylindruria, and hematuria have been reported rarely in patients receiving ciprofloxacin.
Crystalluria generally occurs in patients with alkaline urine who receive high dosage of the drug, and has not been associated with changes in renal function. The risk of crystal formation and crystalluria in patients receiving usual recommended dosages of the drug (250-750 mg) is low if urine pH is within the usual range (i.e., less than 6.8). Patients receiving the drug, particularly at relatively high dosages, should maintain adequate fluid intake; in addition, alkaline urine should be avoided. (See Cautions: Precautions and Contraindications.) Crystalluria, sometimes associated with nephropathy, occurs in animals receiving ciprofloxacin.
This may be related to the fact that urine of test animals (e.g., rats, monkeys) is predominantly alkaline. In studies in rhesus monkeys, crystalluria (without evidence of nephropathy) has occurred after a single 30 mg/kg oral dose of ciprofloxacin. Nephropathy did not occur when these monkeys received 6 months of ciprofloxacin therapy at a dosage of 30 mg/kg daily; however, nephropathy occurred after 6 months of therapy at a dosage of 90 mg/kg daily.
Musculoskeletal Effects
Arthralgia, joint or back pain, joint inflammation, joint stiffness, achiness, vasculitis, neck or chest pain, and flare-up of gout have been reported in less than 1% of patients receiving ciprofloxacin. Achilles and other tendon ruptures that required surgical repair or resulted in prolonged disability have been reported in patients receiving fluoroquinolones, including ciprofloxacin.
Ciprofloxacin should be discontinued in any patient who experiences pain, inflammation, or rupture of a tendon. Ciprofloxacin, like most other fluoroquinolones (e.g., gatifloxacin, levofloxacin, moxifloxacin, norfloxacin, ofloxacin), causes arthropathy in immature animals of various species. (See Cautions: Pediatric Precautions.) Ciprofloxacin has caused damage to weight-bearing joints in juvenile dogs and rats. In young beagles, ciprofloxacin given in a dosage of 100 mg/kg for 4 weeks caused degenerative articular changes of the knee joint; in a daily dosage of 30 mg/kg, effects on the joint were minimal, although some damage to weight-bearing joints was observed even at the lower dosage. Removal of weight bearing from the joint reduced the lesions, but did not totally prevent them.
Morphologic changes observed in animals with quinolone-induced arthropathies include erosions in joint cartilage accompanied by noninflammatory, cell-free effusion of the joint space; the cartilage is incapable of regeneration and may serve as a site for the development of arthropathy deformans. In addition, breakdown products of the cartilage may irritate the synovia. The relationship of these effects in animals and the rheumatologic symptoms associated with use of ciprofloxacin in humans is unknown.
Hepatic Effects
Increased serum concentrations of AST (SGOT) and ALT (SGPT) have been reported in about 2% and increased serum concentrations of alkaline phosphatase, LDH, bilirubin, and Gamma-glutamyltransferase (GGT, gamma-glutamyl transpeptidase, GGTP) have been reported in less than 1% of patients receiving the drug. In addition, fulminant and occasionally fatal hepatic failure has occurred rarely in patients receiving ciprofloxacin.
Hematologic Effects
Eosinophilia, leukopenia, neutropenia, increased or decreased platelet count, and pancytopenia have been reported in less than 1% of patients receiving ciprofloxacin.Anemia, decreased hemoglobin, increased monocytes, leukocytosis, and bleeding diathesis have been reported in less than 1% of patients receiving the drug. In at least one patient, decreased hemoglobin was associated with GI bleeding, although there was no evidence of such bleeding in some other patients with hemoglobin reductions. In addition, transient acquired von Willebrand’s disease has been reported rarely in patients receiving ciprofloxacin; factor VIII concentration returned to normal values several months (i.e., 5-6 months) following discontinuance of the drug in these patients.
Cardiovascular Effects
Palpitation, atrial flutter, ventricular ectopy, syncope, hypertension, angina pectoris, chest pain, myocardial infarction, cardiopulmonary arrest, and cerebral thrombosis have been reported in less than 1% of patients receiving ciprofloxacin.
Local Effects
Local adverse effects have been reported at the site of infusion following IV administration of ciprofloxacin. These reactions generally resolve rapidly after completion of the infusion and have been reported most frequently when IV infusions of the drug were given over 30 minutes or less. The manufacturer states that adverse local reactions to IV ciprofloxacin do not contraindicate subsequent IV administration of the drug, unless the reactions recur or worsen.
Other Adverse Effects
Epistaxis, laryngeal or pulmonary edema, hiccups, hemoptysis, dyspnea, bronchospasm, and pulmonary embolism have been reported in less than 1% of patients receiving ciprofloxacin. Blurred vision, disturbed vision (e.g., change in color perception, overbrightness of lights), decreased visual acuity, diplopia, and eye pain have been reported in less than 1% of patients receiving ciprofloxacin. Although reported with some other quinolones, there has been no evidence of ocular toxicity in animal studies using ciprofloxacin. Tinnitus, anosmia, increased serum amylase, decreased blood glucose, and increased serum uric acid concentrations have been reported rarely (i.e., in less than 0.1% of patients).
Precautions and Contraindications
Crystalluria has been reported rarely in patients receiving ciprofloxacin. Although crystalluria is not expected to occur under usual conditions with the usual recommended dosages of the drug, patients should be instructed to drink sufficient quantities of fluids to ensure proper hydration and adequate urinary output during ciprofloxacin therapy. Measures also should be taken to avoid alkaline urine, and the usual recommended dosage of the drug should not be exceeded.
Because ciprofloxacin, like other quinolones, may cause CNS stimulation that potentially could result in tremor, restlessness, lightheadedness, mental confusion, toxic psychosis, and/or seizures, the drug should be used with caution in patients with known or suspected CNS disorders (e.g., severe cerebral arteriosclerosis, seizure disorders) that predispose to seizures or lower the seizure threshold and should be used with caution in the presence of other factors (e.g., certain drug therapies, renal dysfunction) that predispose to seizures or lower the seizure threshold. Patients should be advised that ciprofloxacin may cause dizziness or lightheadedness, and their individual susceptibility to these adverse effects should be determined before operating a motor vehicle or machinery or engaging in activities requiring mental alertness and coordination.
Patients receiving a theophylline derivative or caffeine concomitantly also may be at increased risk of these CNS effects. In addition, serious and fatal reactions, including cardiac arrest, seizures, status epilepticus, and respiratory failure, have been reported during concurrent theophylline and ciprofloxacin therapy. (See Drug Interactions: Xanthine Derivatives.) Patients receiving ciprofloxacin should be advised to discontinue the drug and inform their physician if they experience pain, inflammation, or rupture of a tendon and to rest and refrain from exercise.
As with other anti-infectives, use of ciprofloxacin may result in overgrowth of nonsusceptible organisms, especially enterococci or Candida. Resistant strains of some organisms (e.g., Pseudomonas aeruginosa, staphylococci) have developed during ciprofloxacin therapy. Careful monitoring of the patient and periodic in vitro susceptibility tests are essential. If superinfection occurs, appropriate therapy should be instituted. Doses and/or frequency of administration of ciprofloxacin IV, conventional tablets, or oral suspension should be decreased in patients with severe renal impairment since serum concentrations of the drug are higher and prolonged in these patients compared with patients with normal renal function.
The manufacturer recommends that organ system function, including renal, hepatic, and hematopoietic, be monitored periodically during prolonged ciprofloxacin therapy. Patients receiving ciprofloxacin should be advised to avoid excessive exposure to sunlight or artificial ultraviolet light and to discontinue therapy if phototoxicity occurs. Moderate to severe phototoxicity manifested as an exaggerated sunburn reaction has been reported during exposure to direct sunlight in patients receiving some fluoroquinolones (lemofloxacin, ofloxacin, sparfloxacin).
Ciprofloxacin, like other quinolones, can cause serious, potentially fatal hypersensitivity reactions, occasionally following the initial dose. (See Cautions: Dermatologic and Sensitivity Reactions.) Patients receiving ciprofloxacin should be advised of this possibility and instructed to discontinue the drug and contact their physician at the first sign of rash or any other sign of hypersensitivity. Ciprofloxacin is contraindicated in patients with a history of hypersensitivity to the drug or to other quinolones.
Pediatric Precautions
Anthrax
Ciprofloxacin may be used in children for inhalational anthrax (postexposure) to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis spores, and the CDC and other experts (US Working Group on Civilian Biodefense) currently recommend that initial treatment of inhalational or systemic (including GI and oropharyngeal) should consist of either IV ciprofloxacin or doxycycline plus 1 or 2 additional anti-infectives. Because of potential adverse effects from prolonged use of ciprofloxacin in infants and children, amoxicillin is an option for completion of the remaining 60 days of therapy (i.e., after an initial 14-21 or 7-10 days of multiple-drug therapy for inhalational or uncomplicated cutaneous anthrax, respectively, that included ciprofloxacin or doxycycline) when susceptibility to penicillin is known; amoxicillin is not recommended for initial treatment. Amoxicillin also can be considered as an alternative to ciprofloxacin for postexposure prophylaxis when there are concerns about the potential adverse effects of prolonged quinolone therapy in children.
Other Infections
Because ciprofloxacin causes arthropathy in immature animals, the manufacturer states that safety and efficacy of the drug for other indications in children and adolescents younger than 18 years of age have not been established. Some clinicians suggest that quinolones may be used cautiously in adolescents if skeletal growth is complete and that the potential benefits of ciprofloxacin therapy may outweigh the possible risks in certain children 9-18 years of age with serious infections (e.g., cystic fibrosis, typhoid fever) when the causative organism is resistant to other available anti-infectives.
The American Academy of Pediatrics (AAP) states that use of fluoroquinolones (e.g., ciprofloxacin, levofloxacin, lomefloxacin, norfloxacin, ofloxacin, sparfloxacin) in children younger than 18 years of age may be justified in special circumstances; however, the drugs should be used only after careful assessment of the risks and benefits for the individual patient and after these benefits and risks have been explained to the parents or caregivers.
The AAP states that fluoroquinolones may be useful when no other oral agent is available (to avoid use of a parenteral agent) or when the pediatric patient has an infection caused by multidrug-resistant gram-negative bacteria, such as certain strains of Pseudomonas, or Mycobacterium. Therefore, possible uses of fluoroquinolones in pediatric patients include the treatment of urinary tract infections caused by P. aeruginosa or other multidrug-resistant gram-negative bacteria, chronic suppurative otitis media or malignant otitis externa, chronic osteomyelitis, exacerbation of cystic fibrosis, mycobacterial infection, or other gram-negative bacterial infections in immunocompromised patients when prolonged oral therapy is desired.
Ciprofloxacin has been used in children with cystic fibrosis (see Uses: Lower Respiratory Tract Infections), but transient arthropathy has occurred occasionally in such children. In at least one 16-year-old child, arthropathy was associated with administration of relatively high dosages of the drug (750 mg twice daily) for several weeks. Oral or IV654, 655, 659 ciprofloxacin also has been used in a limited number of children with typhoid fever resistant to other anti-infectives (e.g., ampicillin, amoxicillin, chloramphenicol, co-trimoxazole). Short-term safety data are available from a randomized, double-blind study in children and adolescents 5-17 years of age with cystic fibrosis who received IV ciprofloxacin for the treatment of acute pulmonary exacerbations.
These pediatric patients were randomized to receive IV ciprofloxacin (10 mg/kg every 8 hours) for 1 week followed by oral ciprofloxacin (20 mg/kg every 12 hours) to complete 10-21 days of therapy or a regimen of IV ceftazidime (50 mg/kg every 8 hours) and IV tobramycin (3 mg/kg every 8 hours) given for 10-21 days. Safety was monitored by periodic range of motion examinations and gait assessments; patients were followed for an average of 23 days after completion of therapy (range: 0-93 days). The study was not designed to determine long-term effects or the safety of repeated exposure to ciprofloxacin. Local reactions at the site of injection were reported more frequently in those who received ciprofloxacin (24%) than in those who received ceftazidime and tobramycin (8%), but other adverse effects were similar and occurred with similar frequency in both groups. In the ciprofloxacin group, musculoskeletal adverse effects, decreased range of motion, and arthralgia were reported in 22, 12, and 10%, respectively; in the combination group, these effects were reported in 21, 16, and 11%, respectively.
One pediatric patient in the study developed arthritis of the knee 9 days after a 10-day regimen of ciprofloxacin; clinical symptoms resolved but MRI showed knee effusion without other abnormalities 8 months after treatment. A causative relationship between this event and ciprofloxacin could not be established, particularly since cystic fibrosis patients may develop arthralgias and/or arthritis as part of their underlying disease process. Oral ciprofloxacin caused lameness in immature dogs; histologic evaluation of the weight-bearing joints of these dogs revealed permanent lesions of the cartilage. (See Cautions: Musculoskeletal Effects.) Most other fluoroquinolones also cause erosions of the cartilage in weight-bearing joints and other signs of arthropathy in immature animals of various species.
Geriatric Precautions
Retrospective analysis of 23 multiple-dose controlled clinical studies evaluating ciprofloxacin in over 3500 patients revealed that 25% of patients included in these studies were 65 years of age or older and 10% were 75 years of age or older. Although no overall differences in safety or efficacy were observed between geriatric individuals and younger adults in these studies and other clinical experience revealed no evidence of age-related differences, the possibility that some older patients may exhibit increased sensitivity to the drug cannot be ruled out. Ciprofloxacin is substantially eliminated by the kidney, and the risk of adverse reactions may be greater in patient with impaired renal function.Although dosage of ciprofloxacin does not need to be modified in individuals older than 65 years of age with normal renal function, the greater frequency of decreased renal function observed in the elderly should be considered and dosage carefully selected in geriatric patients; monitoring renal function may be useful in these patients.
Mutagenicity and Carcinogenicity
Ciprofloxacin was not mutagenic in the rat hepatocyte DNA repair assay or dominant lethal or micronucleus tests in mice. Ciprofloxacin was positive for mutagenicity in the mouse lymphoma cell forward mutation assay and rat hepatocyte DNA repair assay; however, the drug was not mutagenic in other in vitro studies, including the Ames microbial (Salmonella) mutagen test with metabolic activation, Escherichia coli DNA repair assay, Chinese hamster V-79 cell HGPRT test, Syrian hamster embryo cell transformation assay, Saccharomyces cerevisiae point mutation assay, and mitotic crossover and gene conversion assays. No evidence of carcinogenic or tumorigenic potential was seen in mice or rats receiving oral ciprofloxacin in a dosage of 700 mg/kg daily or 250 mg/kg daily, respectively, for up to 2 years.
Pregnancy, Fertitlity and Lactation
There are no adequate and controlled studies to date using ciprofloxacin in pregnant women. Since the drug, like most other fluoroquinolones, causes arthropathy in immature animals, ciprofloxacin should not be used in pregnant women except for the treatment or prevention of inhalational anthrax. (See Inhalational Anthrax: Postexposure Prophylaxis, in Uses.)
Reproduction studies in rats and mice using ciprofloxacin dosages up to 6 times the usual human dosage have not revealed evidence of impaired fertility or harm to the fetus. In rabbits, ciprofloxacin dosages of 30 and 100 mg/kg caused adverse GI effects resulting in maternal weight loss and an increased incidence of abortion, but there was no evidence of teratogenicity. IV ciprofloxacin given to rabbits at dosages up to 20 mg/kg has not resulted in maternal toxicity, embryotoxicity, or teratogenicity.
Administration of high dosages (100 mg/kg daily) of some quinolones (e.g., norfloxacin, pefloxacin [not commercially available in the US] and pipemidic acid [not commercially available in the US]) has been associated with impaired spermatogenesis and/or testicular damage (atrophy in rats and dogs) in chronic (for 3 months or longer) toxicity studies. Ciprofloxacin has been shown to distribute into milk. Because of the potential for serious adverse effects of ciprofloxacin in nursing infants, a decision should be made whether to discontinue nursing or the drug, taking into account the importance of the drug to the woman.
However, the AAP considers ciprofloxacin to be usually compatible with breast-feeding since the amount of the fluoroquinolone potentially absorbed by nursing infants would be small and no observable change in infants associated with such exposure has been reported to date.
Because the long-term safety of prolonged exposure of nursing infants (e.g., during a 60-day regimen for anthrax) to breast milk from ciprofloxacin-treated women currently is not known, the CDC recommends that lactating women who are concerned about the use of ciprofloxacin during anthrax prophylaxis consider expressing and then discarding their breast milk so that breast-feeding can be resumed once anti-infective prophylaxis is complete. Decisions about anti-infective choice and continuation of breast-feeding should be made by the woman and her and the infant’s clinicians, taking into consideration the efficacy of the anti-infective, safety for the infant, and benefits of breast-feeding.
Ciprofloxacin Hydrochloride: Drug Interactions
Antacids
Antacids containing magnesium, aluminum, or calcium decrease absorption of oral ciprofloxacin, resulting in decreased serum and urine concentrations of the anti-infective agent. Serum ciprofloxacin concentrations generally are decreased by 14-50%, but may be decreased up to 90%, in patients receiving an antacid concomitantly; anti-infective treatment failure may occur as a result of reduced quinolone absorption in these patients.
The mechanism of this interaction has not been fully elucidated to date, but magnesium, aluminum, and other divalent ions may bind to, and form insoluble complexes with, quinolones in the GI tract. The manufacturer states that ciprofloxacin extended-release tablets, conventional tablets, or oral suspension should be administered at least 2 hours before or 6 hours after antacids containing magnesium or aluminum.
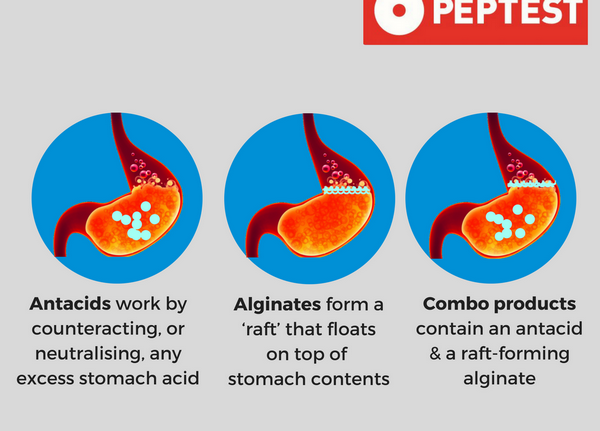
Some clinicians suggest that patients be instructed not to ingest antacids containing magnesium, aluminum, or calcium concomitantly with or within 2-4 hours of a ciprofloxacin dose; however, other clinicians state that these antacids should not be used in patients receiving ciprofloxacin and that ciprofloxacin probably should not be used in patients with renal failure who require aluminum hydroxide or aluminum carbonate for intestinal binding of phosphate.
Aminoglycosides
The antibacterial activities of ciprofloxacin and aminoglycosides have been additive or synergistic in vitro against some strains of Enterobacteriaceae and Pseudomonas aeruginosa. However, synergism between the drugs is unpredictable, and indifference generally occurs when ciprofloxacin is used in conjunction with amikacin, gentamicin, or tobramycin against Ps. aeruginosa or Enterobacteriaceae. Indifference also generally occurs when the drug is used in conjunction with tobramycin against Acinetobacter.
B-Lactam
Antibiotics An additive or synergistic effect has occurred occasionally in vitro against some strains of Ps. aeruginosa and Ps. maltophila when ciprofloxacin was used concomitantly with an extended-spectrum penicillin (e.g., mezlocillin, piperacillin). Indifference generally occurs when ciprofloxacin is used in conjunction with an extended-spectrum penicillin against Enterobacteriaceae. Ciprofloxacin used in conjunction with imipenem, cefoxitin, or a cephalosporin (e.g., cefotaxime, ceftazidime, ceftizoxime) has been reported to be additive or synergistic against some strains of Ps. aeruginosa or Enterobacteriaceae; however, these combinations generally are indifferent rather than additive or synergistic against these bacteria. Although the clinical importance has not been determined, ciprofloxacin used in conjunction with cefotaxime in vitro resulted in a synergistic effect against many strains of Bacteroides fragilis tested; antagonism did not occur.
Didanosine
Concomitant use of oral ciprofloxacin and didanosine administered as either chewable/dispersible buffered tablets or the pediatric powder for oral solution admixed with antacid may decrease absorption of ciprofloxacin resulting in decreased serum and urine concentrations of the quinolone. The manufacturer states that ciprofloxacin extended-release tablets, conventional tablets, or oral suspension should be administered at least 2 hours before or 6 hours after these didanosine preparations.
Other Anti-infectives
The combination of ciprofloxacin and clindamycin has been synergistic in vitro against many strains of Peptostreptococcus, Lactobacillus, and B. fragilis tested. Synergism does not occur in vitro when ciprofloxacin is used in conjunction with vancomycin against Staphylococcus epidermidis, S. aureus (including oxacillin-resistant S. aureus), Corynebacterium, or Listeria monocytogenes. In vitro, the combination of ciprofloxacin and rifampin generally is indifferent against S. aureus; however, antagonism also has been reported rarely.
Probenecid
Concomitant administration of probenecid interferes with renal tubular secretion of ciprofloxacin, resulting in a 50% decrease in renal clearance of ciprofloxacin, a 50% increase in systemic ciprofloxacin concentrations, and a prolonged serum half-life of the drug. This effect should be considered in patients receiving the drugs concomitantly.
Cimetidine, Ranitidine, and Sucralfate
Concomitant sucralfate, presumably because of its aluminum content, decreases GI absorption of ciprofloxacin and may result in a substantial (e.g., 50%) decrease in serum concentrations of the anti-infective agent. Patients should be instructed to take ciprofloxacin extended-release tablets, conventional tablets, or oral suspension at least 2 hours before or 6 hours after sucralfate. Concomitant cimetidine or ranitidine does not appear to alter GI absorption of ciprofloxacin.
Coumarin Anticoagulants
Initiation of oral ciprofloxacin therapy in at least one patient stabilized on warfarin has resulted in prolongation of the prothrombin time and hematemesis. Concomitant use of some other fluoroquinolones (e.g., norfloxacin) in patients receiving coumarin anticoagulants also has resulted in increased prothrombin times. The mechanism of this interaction has not been determined to date, but ciprofloxacin may displace the anticoagulants from serum albumin binding sites. Ciprofloxacin should be administered with caution in patients receiving a coumarin anticoagulant.
Iron, Multivitamins, and Mineral Supplements
Oral multivitamin and mineral supplements containing divalent or trivalent cations such as calcium, iron, or zinc may interfere with oral absorption of ciprofloxacin resulting in decreased serum and urine concentrations of the quinolone. Therefore, these multivitamins and/or mineral supplements should not be ingested concomitantly with ciprofloxacin. The manufacturer states that ciprofloxacin extended-release tablets, conventional tablets, or oral suspension should be administered at least 2 hours before or 6 hours after preparations containing calcium, iron, or zinc.
Xanthine Derivatives
Concomitant administration of ciprofloxacin in patients receiving a theophylline derivative may result in higher and prolonged serum theophylline concentrations and may increase the risk of theophylline-related adverse effects. Alterations in theophylline pharmacokinetics have shown considerable interindividual variation, with serum theophylline concentrations reportedly increasing by 17-254% and theophylline clearance decreasing by 18-112% following initiation of ciprofloxacin.
Generally, however, reductions in theophylline clearance induced by ciprofloxacin have averaged 20-35%. This effect also has been reported with some other quinolones (e.g., norfloxacin); there have been conflicting reports concerning the degree to which norfloxacin affects the pharmacokinetics of theophylline. Alterations in theophylline pharmacokinetics may be related to inhibition of metabolism in the liver by the 4-oxo metabolites of these quinolones. However, the potential contribution of the 4-oxo metabolites to this interaction has not been fully elucidated, and there is some evidence that, while formation of these metabolites may correlate with inhibition of theophylline metabolism, the 4-oxo metabolites themselves may not be responsible for the observed inhibition.
Theophyllines do not appear to affect the pharmacokinetics of quinolones. However, there is limited in vitro evidence that theophyllines may potentiate quinolone-induced inhibition of aminobutyric acid (GABA), thus possibly potentiating CNS stimulation. Serious and fatal reactions have occurred in patients receiving ciprofloxacin and theophylline concomitantly.
Adverse reactions reported during concomitant therapy with the drugs include nausea, vomiting, dizziness, headache, tremor, restlessness, agitation, confusion, seizures (including status epilepticus), hallucinations, tachycardia, cardiac arrest, respiratory failure, and palpitations and apparently occurred as the result of increased serum theophylline concentrations; death in at least one patient was associated with seizures and atrial fibrillation during concomitant therapy with the drugs. While similar effects also have been reported in theophylline-treated patients who were not receiving ciprofloxacin concomitantly, the possibility that such toxicity may have been potentiated by ciprofloxacin cannot be excluded.
Because of the risk of toxicity if plasma theophylline concentrations are increased, concomitant use of ciprofloxacin and a theophylline derivative should be avoided if possible. If the drugs must be given concomitantly, plasma theophylline concentrations should be monitored, the patient observed for manifestations of theophylline toxicity, and appropriate theophylline dosage adjustments made as needed, especially in geriatric patients. The need for theophylline dosage adjustment also should be considered when ciprofloxacin is discontinued since subtherapeutic concentrations may occur.
Although the clinical importance has not been determined to date, concomitant ciprofloxacin prolongs the elimination half-life of caffeine and decreases its volume of distribution and total body clearance.
Patients receiving ciprofloxacin should be advised that regular consumption of large quantities of coffee, tea, or caffeine-containing soft drinks or drugs during therapy with the anti-infective may result in exaggerated or prolonged effects of caffeine. If excessive cardiac or CNS stimulation (e.g., nervousness, insomnia, anxiety, tachycardia) occurs, caffeine intake should be restricted. In addition, caffeine intake should be restricted during ciprofloxacin therapy in patients at risk of adverse effects from CNS or cardiac stimulation.
Other Drugs
Although concomitant administration of ciprofloxacin extended-release tablets (a single 1-g dose; twice the usually recommended dosage) and omeprazole (40 mg once daily for 3 days) in healthy individuals reduced peak plasma concentrations and AUC of ciprofloxacin by about 20%, the interaction was not considered clinically important. Concomitant use of ciprofloxacin and phenytoin has resulted in altered serum concentrations of phenytoin; caution is advised. Severe hypoglycemia has occurred rarely in patients receiving ciprofloxacin and glyburide.
Although the clinical importance has not been determined and further study is needed to evaluate the interactions, concomitant administration of metoclopramide reportedly enhances the rate of GI absorption of ciprofloxacin and concomitant administration of antimuscarinics (e.g., scopolamine, pirenzepine) delays GI absorption of the anti-infective. Although the potential for interaction has not been fully elucidated, ciprofloxacin should be used cautiously in patients receiving drugs that depend on oxidative metabolism in the liver for elimination, particularly those with a narrow therapeutic range, since experience with xanthine derivatives indicates that such interactions may be possible. (See Drug Interactions: Xanthine Derivatives.)
Acute renal failure occurred within 4 days after initiation of ciprofloxacin in a patient receiving cyclosporine maintenance therapy. The mechanism of this potential interaction has not been elucidated, but could involve synergistic nephrotoxic effects of the drugs and/or interference by ciprofloxacin of cyclosporine metabolism. It has been suggested that concomitant use of ciprofloxacin and a nonsteroidal anti-inflammatory agent (NSAIA) could increase the risk of CNS stimulation (e.g., seizures), but additional study and experience are necessary.
How can i get Ciprofloxacin hydrochloride online over the counter?
You can buy Ciprofloxacin hydrochloride OTC in online drugstore with low cost.
Delivery
Australia, Canada, Mexico, New Zealand, USA, Europe [Belgium, France, Norway, Holland, Ireland, Spain, Switzerland, Great Britain (UK), Italy] and etc.