Dirithromycin is a semisynthetic macrolide antibiotic that is structurally and pharmacologically related to erythromycin. Dirithromycin is hydrolyzed nonenzymatically during intestinal absorption almost entirely to erythromycyclamine, which is microbiologically active. Although unchanged dirithromycin and its active metabolite generally appear to exhibit similar in vitro activity against susceptible microorganisms, the contribution of unchanged drug to measured activity remains unclear since dirithromycin is readily hydrolyzed in vitro to the active metabolite. Compared with erythromycin, structural differences in dirithromycin are thought to result in resistance to acid degradation to inactive metabolites in the stomach, improved GI tolerance, improved tissue penetration secondary to increased lipophilicity, decreased potential to interact with other drugs metabolized by the cytochrome P-450 enzyme system, and increased elimination half-life. Dirithromycin and erythromycyclamine generally have microbiologic activity similar to that of erythromycin in vitro against aerobic bacteria, Helicobacter, Mycoplasma, and Chlamydia. However, dirithromycin and/or its metabolite generally are less active than erythromycin in vitro against Haemophilus influenzae and anaerobic bacteria, including Clostridium difficile, Bacteroides, Peptococcus, Peptostreptococcus, and Propionibacterium acnes, and less active in vitro than erythromycin, azithromycin, or clarithromycin against Legionella pneumophila. SumMon® (see Users Guide). For additional information on this drug until a more detailed monograph is developed and published, the manufacturer’s labeling should be consulted. It is essential that the labeling be consulted for detailed information on the usual cautions, precautions, and contraindications.
Uses
Dirithromycin is used for the treatment of mild to moderate upper and lower respiratory tract and skin and skin structure infections caused by susceptible organisms. Dirithromycin should not be used in patients with known, suspected, or potential bacteremia since serum concentrations achievable with oral therapy are inadequate to provide antibacterial coverage in such infections. As with other anti-infective agents, use of dirithromycin may result in overgrowth of nonsusceptible bacteria or fungi. If superinfection occurs, appropriate therapy should be instituted. While Clostridium difficile-associated diarrhea and colitis (also known as antibiotic-associated pseudomembranous colitis) caused by overgrowth of toxin-producing clostridia has been reported rarely with dirithromycin, it should be considered in the differential diagnosis of patients who develop diarrhea during or following therapy with the drug. Mild cases of colitis may respond to discontinuance of the drug alone, but diagnosis and management of moderate to severe cases should include appropriate bacteriologic studies and treatment with fluid, electrolyte, and protein supplementation as indicated. If colitis is severe or is not relieved by discontinuance of the drug, appropriate anti-infective therapy (e.g., oral metronidazole or vancomycin) should be administered.
Pharyngitis and Tonsillitis
Dirithromycin is used for the treatment of pharyngitis and tonsillitis caused by Streptococcus pyogenes (group A b-hemolytic streptococci) in adults and children 12 years of age or older. Although dirithromycin generally is effective in eradicating S. pyogenes from the nasopharynx, data establishing the efficacy of the drug in the subsequent prevention of rheumatic fever currently are not available.
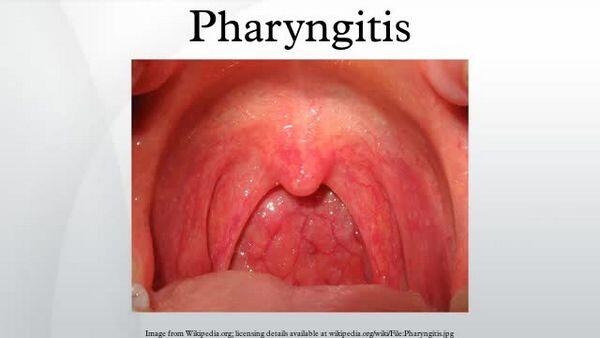
Because penicillin has a narrow spectrum of activity, is inexpensive, and generally is effective, the US Centers for Disease Control and Prevention (CDC), 17 American Academy of Pediatrics (AAP), American Academy of Family Physicians (AAFP), Infectious Diseases Society of America (IDSA), American Heart Association (AHA), American College of Physicians-American Society of Internal Medicine (ACP-ASIM), and others consider natural penicillins (i.e., 10 days of oral penicillin V or a single IM dose of penicillin G benzathine) the treatment of choice for streptococcal pharyngitis and tonsillitis and prevention of initial attacks (primary prevention) of rheumatic fever, although oral amoxicillin often is used instead of penicillin V in small children because of a more acceptable taste. Other anti-infectives (e.g., oral cephalosporins, oral macrolides) generally are considered alternatives.
Respiratory Tract Infections
Dirithromycin is used for the treatment of acute bacterial exacerbations of chronic bronchitis caused by Haemophilus influenzae, Streptococcus pneumoniae, or Moraxella (Branhamella) catarrhalis in patients 12 years of age or older. Dirithromycin is used for the treatment of secondary bacterial infection of acute bronchitis caused by M. catarrhalis or S. pneumoniae in patients 12 years of age or older. Dirithromycin also is used for the treatment of mild to moderately severe community-acquired pneumonia caused by S. pneumoniae, Mycoplasma pneumoniae, or Legionella pneumophila in patients 12 years of age or older whose disease is appropriate for outpatient oral therapy. The drug should not be used if the potential for coexisting bacteremia exists.
Skin and Skin Structure Infections
Dirithromycin is used in patients 12 years of age and older for the treatment of uncomplicated mild to moderate skin and skin structure infections caused by susceptible Staphylococcus aureus (methicillin-susceptible strains) or S. pyogenes; abscesses usually require concurrent surgical drainage.
Dosage and Administration
Administration
Dirithromycin is administered orally as enteric-coated tablets. Enteric coating protects the drug from hydrolysis in the stomach, thus enhancing delivery to the small intestine for absorption. Dirithromycin should be administered with food or within 1 hour after a meal. Commercially available dirithromycin enteric-coated tablets should be swallowed intact and should not be subdivided, chewed, or crushed.
Dosage
Safety and efficacy of dirithromycin in pediatric patients younger than 12 years of age have not been established. Dirithromycin dosage adjustment based solely on age does not appear necessary for geriatric patients since clinically important alterations in pharmacokinetics of the drug in such patients with normal renal and hepatic function have not been observed. In addition, clinical safety and efficacy of dirithromycin were comparable to those in younger adults when the drug was administered at the usual dosage of 500 mg once daily in clinical trials in geriatric adults 65 years of age and older.
Pharyngitis and Tonsillitis
For the treatment of mild to moderate pharyngitis or tonsillitis, the usual dosage of dirithromycin in adults and pediatric patients 12 years of age and older is 500 mg once daily for 10 days; the longer duration of therapy relative to the treatment of bronchitis or skin infections is intended to reduce the risk of recurrence and rheumatic fever complications.
Respiratory Tract Infections
The usual oral dosage of dirithromycin for the treatment of mild to moderate acute exacerbations of chronic bronchitis in adults and pediatric patients 12 years of age or older is 500 mg once daily for 5-7 days. The usual oral dosage of dirithromycin for the treatment of secondary bacterial infection of acute bronchitis in adults and pediatric patients 12 years of age or older is 500 mg once daily for 7 days. The usual oral dosage of dirithromycin for the treatment of mild to moderate community-acquired pneumonia in adults and pediatric patients 12 years of age and older is 500 mg once daily for 14 days.
Skin and Skin Structure Infections
The usual oral dosage of dirithromycin for the treatment of uncomplicated skin and skin structure infections in adults and pediatric patients 12 years of age or older is 500 mg once daily for 5-7 days.
Dosage in Renal and Hepatic Impairment
Limited data from patients with various degrees of renal failure receiving dirithromycin indicate that peak drug concentrations and area under the concentration-time curve (AUC) may increase and urinary excretion and drug clearance may decrease, but generally only in patients with severe renal insufficiency. Although most clinical trials excluded patients with renal impairment, currently available data indicate that no dosage adjustment should be necessary in patients with renal impairment, including in hemodialyzed patients. The effects of hepatic impairment on the elimination of dirithromycin have not been evaluated fully; however, the drug and its active metabolite appear to be eliminated principally via the liver and bile. In patients with mild hepatic impairment (Child’s grade A), no clinically important pharmacokinetic alterations have been observed; therefore, dirithromycin dosage adjustment does not appear necessary in patients with mildly impaired hepatic function. However, because use of dirithromycin in patients with moderate or severe hepatic impairment (Child’s grade B or greater) has not been systematically studied to date, the manufacturer states that the drug should be used in such patients only when absolutely necessary.
Preparations
Dirithromycin Oral Tablets, delayed- 250 mg Dynabac®, (with benzyl alcohol release (enteric and propylene glycol) Muro coated) Dynabac®D5-Pak®, (with benzyl alcohol and propylene glycol; available as a 5-day mnemonic pack of 10 tablets) Muro

