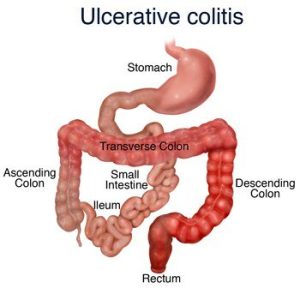
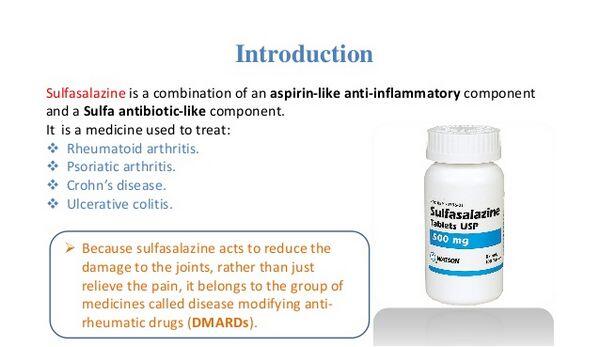
Do you have arthritis or ulcerative colitis? In this case, you may be prescribed to take Sulfasalazine. However, like any other drug, it should be taken with care after you check the instructions and make sure it’s safe for you. Learn more about Sulfasalazine to enjoy health benefits and avoid unexpected side effects.
What is Azulfidine (Sulfasalazine)?
Sulfasalazine is a drug that is produced in the form of tablets for oral intake. Azulfidine is the brand name of the medicine.
Why is Azulfidine Prescribed to Patients? Such diseases as rheumatoid/juvenile arthritis and ulcerative colitis are followed by severe pain. Azulfidine is a painkiller that helps relieve pain symptoms and treats inflammation. In most cases, this medicine is prescribed when the patient has already got treatment with other medications, but they weren’t effective. The action of this drug is directed to remove swelling, which is one of the must-have symptoms of any type of arthritis. Patients who suffer from mild stomachache will benefit from using Azulfidine (Sulfasalazine). Those who have severe pain may be prescribed some additional drugs that can be combined with Sulfasalazine.
Forms and Dosage
| Dosage Form | Strength |
|---|---|
| Sulfasalazine powder | – |
| Pms-Sulfasalazine | 500 mg Tablet |
| Sulfasalazine | 500 mg Tablet |
| Sulfazine | 500 mg Tablet |
| Salazopyrin | 500 mg Tablet |
| Pms-Sulfasalazine Enteric-Coated Tablet | 500 mg |
| Sulfasalazine Enteric Coated Tabs | 500 mg |
| Salazopyrin En-Tabs | 500 mg Enteric-Coated Tablet |
| Azulfidine | 500 mg Tablet |
| Azulfidine entab | 500 mg |
| Azulfidine EN-tabs | 500 mg Enteric Coated Tabs |
How to Take Sulfasalazine?
“How should I take Azulfidine (Sulfasalazine)?” – It’s the question you must ask your healthcare specialist as the dosage may vary. Several factors may affect the dosage regimen. The most important one is your current health condition. If it is severe, you may be prescribed to take a larger dosage. It would help if you informed the doctor about all your health problems, as you may need to decrease or increase the dosage mentioned in the general instructions. Pay attention to the reaction of your body when you take the drug for the first time. Age is also crucial in deciding how much medicine you should take.
Sulfasalazine conventional and delayed-release tablets are administered orally. The daily dosage should be divided into equally divided doses and administered after meals. The delayed-release tablets should be swallowed whole.
Dosage for Ulcerative Colitis
The dosage of Sulfasalazine Azulfidine varies, depending on age and disease.
For the treatment of ulcerative colitis, the interval between doses of sulfasalazine given as conventional or delayed-release tablets should not exceed 8 hours. The response to sulfasalazine in ulcerative colitis patients can be evaluated by clinical criteria (e.g., presence of fever, weight changes, degree and frequency of diarrhea and bleeding) and by sigmoidoscopy and evaluation of biopsy samples.
Continuation of sulfasalazine therapy may be necessary even when clinical symptoms, including diarrhea, have been controlled. When endoscopic examination confirms satisfactory improvement, sulfasalazine dosage may be decreased to a maintenance dosage.
If diarrhea recurs, the dosage should be increased to the previously effective dosage. Patients with ulcerative colitis should be advised that the disease rarely remits completely and that continued use of maintenance dosages of sulfasalazine may decrease the risk of relapse.
The usual initial adult dosage of sulfasalazine given as conventional or delayed-release tablets for treating ulcerative colitis is 3-4 g daily, given in equally divided doses. In some patients, initiating therapy with a dosage of 1-2 g daily may be advantageous to lessen adverse GI effects. Although a dosage as high as 12 g daily has been given, a dosage exceeding 4 g daily is accompanied by an increased incidence of adverse effects.
Some clinicians recommend that dosage exceeding 4 g a day should be avoided unless the serum concentration of total sulfapyridine and the patient’s phenotype are known. The usual adult maintenance dosage is 2 g daily in 4 divided doses. However, some clinicians advocate a lower maintenance dosage of 1-1.5 g daily to prevent adverse effects. The efficacy of maintenance therapy is dose-related. Still, the potential value of dosages of more than 2 g daily must be weighed against the risks of increased adverse effects and the necessity for more careful monitoring of the patient.
When sulfasalazine is given as conventional tablets for treating ulcerative colitis in children 2 years or older, the usual initial dosage is 40-60 mg/kg daily in 3-6 divided doses. The usual maintenance dosage is 30 mg/kg daily in 4 divided doses. When sulfasalazine is given as delayed-release tablets for treating ulcerative colitis in children 6 years or older, the usual initial dosage is 40-60 mg/kg daily in 3-6 divided doses. The usual maintenance dosage is 30 mg/kg daily in 4 divided doses.
Dosage for Rheumatoid Arthritis
The medicine can be taken at the age of 18. For the treatment of rheumatoid arthritis, the interval between doses of sulfasalazine given as delayed-release tablets usually is 12 hours. The usual adult dosage of sulfasalazine given as delayed-release tablets for managing rheumatoid arthritis is 2-3 g daily in equally divided doses. It may be advantageous to initiate therapy with a dosage of 0.5-1 g daily to lessen adverse GI effects. Don’t start with a large dosage of Azulfidine, as you may suffer from stomachache.
| Week of Therapy | Morning Dose (g) | Evening Dose (g) | Total Daily Dose (g) |
|---|---|---|---|
| 1 | 0.5 | 0.5 | 0.5 |
| 2 | 0.5 | 1.0 | 1.0 |
| 3 | 0.5 | 1.0 | 1.5 |
| 4 and beyond | 1.0 | 1.0 | 2.0 |
A response to Sulfasalazine (manifested by improvement in the number and extent of actively inflamed joints) may occur after 4-12 weeks of therapy. Patients receiving sulfasalazine dosages exceeding 2 g daily should be carefully monitored.
Dosage for Juvenile Arthritis
The usual dosage of sulfasalazine given as delayed-release tablets for managing polyarticular course juvenile rheumatoid arthritis in children 6 years of age and older is 30-50 mg/kg daily in 2 equally divided doses; the maximum dosage usually is 2 g daily. To reduce GI intolerance, the manufacturer recommends that sulfasalazine therapy in children be initiated with 1/4 to 1/3 of the planned maintenance dosage and that dosage be increased at weekly intervals until the planned maintenance dosage is achieved (usually at week 4).
Take as Directed
Azulfidine is a drug that doesn’t work fast. Some time is needed for the medicine to start working. You should follow the doctor’s instructions and take the drug as long as it’s necessary to treat your health condition. If it’s severe, then the period of treatment will be extended. Remember that you can’t stop taking the drug when you want; otherwise, you may experience adverse reactions. Your condition may worsen if you decide to stop taking Sulfasalazine without professional consultation.
One more thing to keep in mind: the guarantee of success is the intake of the drug in the prescribed dosage. If you take it at different intervals and don’t follow the instructions, you will unlikely enjoy health benefits. You will notice the desired positive effect from the medicine intake only if the prescribed amount of the medication is taken regularly. The principle of action is that your body accumulates a certain amount of Sulfasalazine to fight the cause of your current health condition.
“How will I know that the drug works for me?” – It’s as easy as ABC. You won’t suffer from joint pain any longer, or it will become milder than before the treatment.
Sulfasalazine Side Effects
Like any other drug, this medicine may cause unwanted reactions. It isn’t a must that you will suffer from side effects when taking it. Still, you need to get acquainted with all the possible adverse reactions you may experience. Some side effects of Azulfidine (Sulfasalazine) are more common, while others take place in rare cases, but they are more serious. Check what side effects the drug has to be well-informed and know what to expect after taking the medication.
Generally, the incidence of severe sulfasalazine-induced adverse effects is low, but minor adverse effects occur frequently. The onset of adverse effects generally occurs within a few days to 12 weeks following initiation of sulfasalazine therapy, especially when the dosage exceeds 4 g daily.
Patients with Ulcerative Colitis
Clinical experience to date indicates that the incidence of sulfasalazine-induced adverse effects in patients with ulcerative colitis is generally similar to that reported in patients with rheumatoid arthritis. The most frequent adverse effects associated with sulfasalazine therapy in patients with ulcerative colitis are anorexia, headache, nausea, vomiting, gastric distress, and reversible oligospermia.
Other adverse effects reported in patients with ulcerative colitis include pruritus, urticaria, rash, fever, Heinz body anemia, hemolytic anemia, and cyanosis. Adverse effects reported in patients with rheumatoid arthritis receiving sulfasalazine include nausea, dyspepsia, headache, abdominal pain, vomiting, fever, dizziness, stomatitis, rash, pruritus, abnormal liver function test values, leukopenia, and thrombocytopenia; reversible immunoglobulin suppression, rarely accompanied by clinical findings, has been observed in sulfasalazine-treated patients with rheumatoid arthritis.
Patients with Rheumatoid Arthritis
It appears that there are no drug-induced adverse effects that are specific to patients with rheumatoid arthritis; however, the rash occurs more frequently in patients with rheumatoid arthritis than in those with ulcerative colitis, occurring in 13 or 3.3% of patients with rheumatoid arthritis or ulcerative colitis, respectively. Most patients with adverse effects (except rashes) have serum total sulfapyridine concentrations exceeding 50 mcg/mL. The ability to acetylate sulfasalazine may influence the onset and severity of adverse effects. In one study, 86% of patients exhibiting adverse effects were slow acetylators of sulfapyridine.
GI Effects
It’s okay if you don’t want to eat when taking Sulfasalazine. Most patients claim that they have lost appetite during treatment with Sulfasalazine medicine. In some cases, it’s connected with the feeling of nausea that may also appear when using Sulfasalazine. Headache and sometimes dizziness are also common side effects that may appear for a short period and disappear without any special drug treatment.
Nausea, vomiting, gastric distress, diarrhea, and anorexia occur frequently in patients receiving sulfasalazine. The manufacturers suggest that GI intolerance occurring after the first few doses of sulfasalazine is probably caused by mucosal irritation and may be alleviated by distributing the total daily dose more evenly over the day or by giving enteric-coated tablets; however, there have been no definitive studies comparing the toxicity of enteric-coated and uncoated tablets.
Symptoms occurring after the first few days of sulfasalazine therapy are probably due to high serum concentrations of total sulfapyridine. They may be alleviated by halving the dose and gradually increasing it over several days. If symptoms persist, the drug should be discontinued for 5-7 days, and therapy should be reinstituted at a lower daily dosage.
There have been isolated reports of enteric-coated sulfasalazine tablets passing intact through the GI tract of some patients, possibly because of a lack of intestinal esterases capable of disintegrating the enteric coating. Additional enteric-coated tablets should not be administered if this occurs.
Sensitivity Reactions
You may also notice that your body itches. It’s the body’s allergic reaction.
If a hypersensitivity reaction occurs during sulfasalazine therapy, the drug should be discontinued immediately. Desensitization to sulfasalazine is used when reinstitution of therapy with the drug is considered necessary in a patient who has had a hypersensitivity reaction to the drug; however, desensitization should not be attempted in patients with a history of agranulocytosis, toxic epidermal necrolysis, fibrosing alveolitis, or anaphylactoid reaction while receiving sulfasalazine.
Specialized references should be consulted for specific information on desensitization procedures and dosage. Although various desensitization procedures have been reported to be effective, many regimens use an initial sulfasalazine dosage of 50-250 mg daily, which is then doubled every 4-7 days until the desired therapeutic dosage is attained. If symptoms of sensitivity recur, the drug should be discontinued.
If you have a fever, a pale color of your skin, spots of unknown origin, other skin problems, sore throat, or sudden discomfort when urinating – call the doctor immediately.
Other Adverse Effects
A few cases of pulmonary eosinophilia and at least one fatality from fibrosing alveolitis have been reported in patients receiving sulfasalazine. Sulfasalazine may impart an orange-yellow color to alkaline urine and skin.
Some men notice that the amount of sperm has decreased after they have started to use the medication. Don’t worry if this happens to you, as it’s just one of the side effects. After the treatment with Sulfasalazine Azulfidine, the sperm count will return to the norm.
You should consider that it is impossible to predict what side effects of Azulfidine you will experience as the body’s reaction is individual. The side effects listed here have appeared in patients, but it doesn’t mean you must have any of them; you may have a side effect that hasn’t been listed here.
What Other Drugs Will Affect Sulfasalazine?
It’s essential to check what medications Sulfasalazine interacts with before starting the treatment. Some interactions may have a negative effect on the action of the medication. It won’t show the desired effect, or it will take longer to start working. Check how Sulfasalazine works in combination with the following drugs.
- Folic acid. Azulfidine prevents Folic Acid from complete absorption. Vitamin B9 should be taken in a larger dosage if you take this drug simultaneously with the vitamin supplement. However, you shouldn’t increase the dosage without the doctor’s consultation.
- Heart medicines. If your doctor has prescribed you to take heart drugs, make sure that they don’t interact with Sulfasalazine; otherwise, they may not be as effective. For example, heart medication like digoxin shouldn’t be combined with Azulfidine. You’ll get the lower dosage of the heart drug as Sulfasalazine will influence its absorption level.
- Disease-modifying antirheumatic drugs. Some of the drugs that refer to the category of disease-modifying antirheumatic drugs may intensify adverse reactions in the body when taking Sulfasalazine Azulfidine simultaneously. Most patients report that they have had nausea when taking Methotrexate together with Sulfasalazine Azulfidine.
The drugs mentioned above are just examples of medications that can interact with Azulfidine. The list of drugs can be longer. You’re strongly recommended to inform your doctor if you take some other drugs simultaneously with Sulfasalazine to avoid any unexpected and life-threatening side effects. Your pharmacist will decide whether you need to continue taking the drug or if it’s possible to take Azulfidine after you finish the treatment course with some other medication.
Overdose
Make sure that you haven’t overdosed on the drug. If you take a larger dosage, you risk having nausea, stomachache, and other unwanted effects. In case you take too much of the drug, you should go to an emergency room, as large doses of Azulfidine are poisonous. Don’t waste time; go to the doctor immediately when you understand you’ve taken a larger dosage.
Missed Dose
Many people take an extra dosage when they understand they missed it. It’s possible to take it only if you remember the missing dose soon. If it’s almost time for the next intake, you shouldn’t take the exact dosage twice, as it will lead to an overdose.
Important Warnings
Check important warnings to avoid any severe side effects, as some of them can be fatal (lead to death).
Refills
You will receive many refills from your healthcare specialist, who has prescribed you to take Azulfidine. This means that you won’t need to get a new prescription every time the drug is over and you need a new portion.
Clinical Monitoring
Clinical monitoring is a must to ensure the drug has a positive effect on your body. During Azulfidine treatment, you will have to visit your doctor regularly and have the standard blood test. You should consider that the treatment with Sulfasalazine supposes many blood tests at the beginning due to the risk of infection caused by the medicine. Besides blood tests, you should be ready to test how your liver works, as the drug may have a negative effect on this organ. Do you have problems with your kidneys? Then, it would help if you told your doctor about this, and they will make a kidney test to prevent possible side effects the drug may cause.
Your Diet
You can continue eating the same foods as you did. The only thing to consider is that Sulfasalazine influences the absorption of folic acid. That’s why you should ask the doctor whether you need to take an extra dosage of folic acid.
Sun Sensitivity
Forget about sunbathing and tanning for the period of treatment with Sulfasalazine. The drug may influence your skin health as it becomes more sensitive to the sun. If you take the medication during the summer, you should use some creams with UV protection.
Is Sulfasalazine Safe to Use During Pregnancy or While Breastfeeding?
Reproduction studies in rats and rabbits using sulfasalazine dosages up to 6 times the usual human dosage have not revealed evidence of harm to the fetus. Sulfasalazine has been used for the treatment of inflammatory bowel disease, including Crohn’s disease and ulcerative colitis, during pregnancy.
Although fetal abnormalities occasionally have been reported in infants born to women with inflammatory bowel disease who received sulfasalazine alone or combined with corticosteroids during pregnancy, most evidence indicates that sulfasalazine is not associated with a substantial risk of teratogenicity and that the potential benefits of therapy with the drug generally appear to outweigh the possible risks in pregnant women with this disease.
Although most experience with the use of sulfasalazine in pregnancy has been in women with inflammatory bowel disease, the safety of the drug in pregnant women with rheumatoid arthritis is not expected to differ from that in inflammatory bowel disease. Sulfasalazine therapy generally can be continued in pregnant women with rheumatoid arthritis. Some clinicians consider sulfasalazine the disease-modifying antirheumatic drug (DMARD) of choice in women who are planning to become pregnant or who are pregnant. The risk of sulfasalazine-induced kernicterus in neonates born to women who received the drug during the last trimester appears to be low. Agranulocytosis has been reported in a neonate whose mother received sulfasalazine and corticosteroid therapy throughout pregnancy.
The effect of the drug on subsequent growth development and functional maturation in children whose mothers received sulfasalazine during pregnancy has not been determined. Because there are no adequate and controlled studies to date using sulfasalazine in pregnant women, the drug should be used during pregnancy only when clearly needed.
Impairment of male fertility was observed in reproduction studies in rats using sulfasalazine dosages of 800 mg/kg daily. Oligospermia, abnormal sperm forms, impaired sperm motility, and infertility have occurred in men receiving sulfasalazine; however, these effects appear to be reversible following discontinuance of the drug. These effects appear to be caused by sulfapyridine, not 5-aminosalicylic acid (mesalamine), on sperm maturation.
Are you breastfeeding and want to take Sulfasalazine Azulfidine? You should know that the baby will get the dosage of the medicine together with milk. You shouldn’t take the drug without a doctor’s prescription. Otherwise, your baby will suffer from side effects that aren’t fatal but are still severe and not safe for the baby.
Mutagenicity and Carcinogenicity
In carcinogenicity studies in rats and mice, an increased incidence of urinary bladder transitional cell papillomas was observed in male rats, an increased incidence of urinary bladder transitional cell papilloma of the kidney was observed in female rats, and an increased incidence of hepatocellular adenoma or carcinoma was observed in male and female mice.
Allergy Warning
It is essential to inform the doctor about any allergic reactions you have ever had when you were taking drugs that contain the same active ingredient as Azulfidine. You should take the possibility of an allergic reaction seriously, as it may become the reason for death. What to do if you don’t know for sure whether you have an allergy to sulfa drugs or not? To be on the safe side, take the small dosage and watch the body’s reaction. Call emergency services immediately if you notice that it’s hard to breathe or you have a sore throat.
Pediatric Precautions
The safety and efficacy of sulfasalazine in children younger than 2 years of age with ulcerative colitis have not been established. The safety and efficacy of sulfasalazine for managing polyarticular course juvenile rheumatoid arthritis in children 6-16 years of age is supported by evidence from adequate and well-controlled studies in adults.
Warnings for People with Certain Health Conditions
- Patients with asthma.
- Patients with porphyria.
Warnings refer to people of the groups mentioned above. If patients suffering from the mentioned conditions use Azulfidine, they may have unwanted adverse body reactions, and their condition may worsen.
Storage
You should store Azulfidine in the room, not in the refrigerator and in places with a damp climate. The optimal temperature required for storing the medicine is 20-25 C.
Other Names of Sulfasalazine
Sulfasalazine, Sulphasalazine, Azulfidine EN-tabs, Sulfazine, Salazopyrin, Salazopyrin En-tabs, Azopyrin, Salazosulfapyridine, Salazosulfapyridinum, Salicylazosulfapyridine, Sulfasalazin, Sulfasalazina.
Therapeutic Classes of Sulfasalazine
Anti-Infective Agents, Antirheumatic Agents, Gastrointestinal Agents, Sulfonamides
Delivery
Australia, Canada, Mexico, New Zealand, USA, Europe (Belgium, France, Norway, Holland, Ireland, Spain, Switzerland, Great Britain (UK), Italy), etc.