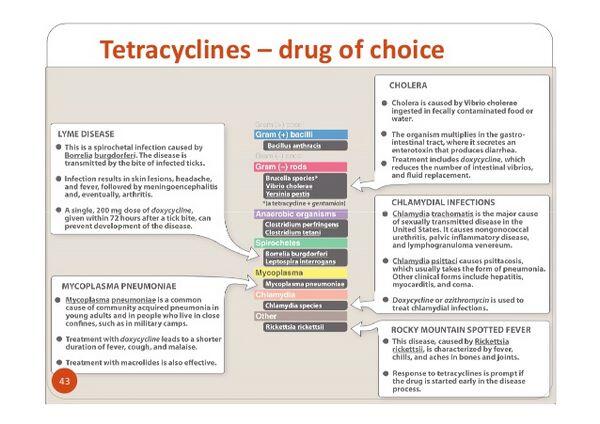
Tetracyclines are used principally in the treatment of infections caused by susceptible Rickettsia, Chlamydia, Mycoplasma, and a variety of uncommon gram-negative and gram-positive bacteria.
Because of the development of resistance, tetracyclines are rarely used for the treatment of infections caused by common gram-negative or gram-positive bacteria unless other appropriate anti-infectives are contraindicated or are ineffective and in vitro susceptibility tests indicate that the causative organisms are susceptible to the drugs. Generally, given a susceptible organism, the currently available tetracyclines are all equally effective when administered in appropriate dosages.
Because minocycline and, to a lesser extent, doxycycline penetrate most body tissues and fluids better than do other currently available tetracyclines, some clinicians prefer these derivatives in the treatment of infections of the CNS, eye, or prostate.
Because of poor CNS penetration, none of the currently available tetracyclines should be used in the treatment of meningitis. Doxycycline generally is the preferred derivative when a tetracycline is indicated in patients with impaired renal function. Because of its low renal clearance, doxycycline may not be as effective as other currently available tetracyclines for the treatment of urinary tract infections in patients with normal or impaired renal function.
Rickettsial Infections
Tetracyclines are used for the treatment of rickettsial infections and are considered drugs of choice for Rocky Mountain spotted fever, epidemic (louse-borne) typhus, Brill-Zinsser disease, endemic (murine) typhus, scrub typhus, Q fever, and rickettsialpox. Some clinicians suggest that doxycycline is the preferred tetracycline for the treatment of Rocky Mountain spotted fever, endemic typhus, epidemic typhus, scrub typhus, trench fever, and Q fever. Although doxycycline alone usually is the drug of choice for the treatment of acute Q fever caused by Coxiella burnetii, doxycycline has been used in conjunction with hydroxychloroquine or a fluoroquinolone (e.g., levofloxacin, ofloxacin) for the treatment of Q fever endocarditis. In one limited study in patients with confirmed C. burnetti infection and chronic endocarditis, a regimen of doxycycline and hydroxychloroquine was associated with a lower relapse rate than a regimen of doxycycline and ofloxacin. Although both regimens require long-term therapy, the mean duration of therapy for cured patients was 55 months for those who received the doxycycline/quinolone regimen compared with 31 months for those who received the doxycycline/hydroxychloroquine regimen. Prolonged therapy (at least 18 months) with the doxycycline and hydroxychloroquine regimen is necessary to prevent relapse. The US Centers for Disease Control and Prevention (CDC ) recommends a 2- to 3-week regimen of doxycycline for the treatment of acute Q fever, a 1-year regimen of doxycycline and hydroxychloroquine for the treatment of acute Q fever in patients with preexisting valvular heart disease (to prevent progression of acute disease to endocarditis), and a 1.5- to 3-year regimen of doxycycline and hydroxychloroquine for the treatment of chronic Q fever.
Chlamydial Infections
Tetracyclines are highly effective in the treatment of most chlamydial infections, including urogenital infections caused by Chlamydia trachomatis, respiratory tract infections caused by C. pneumoniae, respiratory tract infections caused by C. psittaci (psittacosis), and lymphogranuloma venereum caused by invasive serovars of C. trachomatis.
Urogenital Chlamydial Infections in Adults and Adolescents
For the treatment of urogenital chlamydial infections in adults and adolescents, the CDC and some clinicians recommend a single dose of oral azithromycin or a 7-day regimen of oral doxycycline. Alternatively, adults and adolescents with urogenital chlamydial infections can receive a 7-day oral regimen of erythromycin base, erythromycin ethylsuccinate, ofloxacin, or levofloxacin. Results of clinical studies indicate that the single-dose azithromycin and multi-dose doxycycline regimen are equally effective for the treatment of urogenital chlamydial infections when patients are compliant and follow-up encouraged; however, if poor compliance or inability to provide follow-up are a concern, azithromycin may be more cost-effective since the single-dose regimen can be administered under direct supervision. Erythromycin is less effective than either azithromycin or doxycycline and GI effects associated with the drug may discourage patient compliance with the regimen. To maximize compliance with 7-day regimens, the CDC recommends that the drugs be dispensed on site and that the first dose be taken under supervision. Since the azithromycin and doxycycline regimens are highly effective, a test of cure probably is unnecessary in patients who receive one of these regimens unless symptoms persist or reinfection is suspected; however, a test of cure should be considered 3 weeks after completion of an erythromycin regimen. Some studies have demonstrated high rates of infection among women retested for chlamydia after treatment, presumably because of reinfection. In some populations (e.g., adolescents), rescreening women several months after treatment might be effective for detecting further morbidity. Patients being treated for chlamydial infection should be instructed to refer their sexual partner(s) for evaluation and treatment, and to abstain from sexual intercourse for 7 days after single-dose therapy or until completion of a 7-day regimen. In addition, to minimize the risk of reinfection, patients should be instructed to abstain from sexual intercourse until after all their sexual partners are cured. Although the CDC acknowledges that the exposure intervals are somewhat arbitrary, they recommend that individuals who had sexual contact with the chlamydia patient within 60 days before the onset of symptoms or diagnosis in the patient should be evaluated and treated. If the patient reports that the last sexual contact occurred more than 60 days prior to the onset of symptoms or diagnosis, their most recent sexual partner should be treated. Individuals with HIV infection who also are infected with chlamydia should receive the same treatment regimens recommended for other individuals with chlamydial infections.
Urogenital Chlamydial Infections in Children
For the treatment of urogenital chlamydial infections in children who weigh less than 45 kg, the CDC recommends a 14-day regimen of oral erythromycin base or ethylsuccinate. For the treatment of urogenital chlamydial infections in children younger than 8 years of age who weigh at least 45 kg, the CDC recommends a single dose of oral azithromycin; for those 8 years of age and older, the CDC recommends either a single dose of azithromycin or a 7-day regimen of oral doxycycline.
Presumptive Treatment of Chlamydial Infections in Patients with Gonorrhea
Patients infected with N. gonorrhoeae frequently also have coexisting chlamydial and mycoplasmal infection; however, cephalosporins, spectinomycin, and most quinolone regimens used for the treatment of gonorrhea are ineffective for the treatment of these infections. Because of the risks associated with untreated coexisting chlamydial infection, the CDC and most clinicians recommend that patients being treated for uncomplicated gonorrhea or disseminated gonococcal infection also receive an anti-infective regimen effective for presumptive treatment of uncomplicated urogenital chlamydial infection. For presumptive treatment of chlamydia in adults and adolescents being treated for uncomplicated or disseminated gonococcal infections, the CDC recommends use of a single dose of oral azithromycin or a 7-day regimen of oral doxycycline. The strategy of routine administration of a regimen effective against chlamydia in patients being treated for gonococcal infection has been recommended by the CDC for more than 10 years and appears to have resulted in substantial decreases in the prevalence of urogenital chlamydial infection in some populations. In addition, since most N. gonorrhoeae isolated in the US are susceptible to doxycycline and azithromycin, dual therapy possible may delay the development of resistance in N. gonorrhoeae. Since the cost of presumptive treatment of chlamydia is less than the cost of testing for presence of chlamydia, routine dual therapy without chlamydial testing can be cost-effective for populations in which coinfection with chlamydia has been reported in 10-30% of patients with N. gonorrhoeaeinfection. In areas where the rate of coinfection with chlamydia is low and chlamydial testing is widely available, some clinicians may prefer to test for chlamydia rather than treat presumptively; however, presumptive treatment is indicated for patients who may not return for test results.
Trachoma and Inclusion Conjunctivitis
An oral tetracycline (with or without a topical tetracycline, topical erythromycin, or topical sulfacetamide) is used for the treatment of trachoma and inclusion conjunctivitis caused by C. trachomatis in adults and children older than 8 years of age; however, anti-infective therapy may not eliminate C. trachomatis in all cases of chronic trachoma. Inclusion conjunctivitis and trachoma in younger children and neonates and chlamydial infections in pregnant women generally are treated with oral erythromycin.
Lymphogranuloma Venereum
Doxycycline generally is considered the drug of choice for the treatment of lymphogranuloma venereum caused by invasive serotypes of C. trachomatis (serovars L1, L2, L3), and oral erythromycin is considered an alternative regimen for the treatment of the disease. Erythromycin is the preferred regimen for the treatment of lymphogranuloma venereum in pregnant and lactating women. Effective treatment cures the infection and prevents ongoing tissue damage, although tissue reaction can result in scarring. Aspiration of buboes or incision and drainage may be necessary to prevent the formation of inguinal/femoral ulcerations. The CDC recommends that individuals who had sexual contact with the lymphogranuloma venereum patient should be examined, tested for urethral or cervical chlamydial infection, and treated if they had sexual contact with the patient within 30 days prior to onset of symptoms in the patient. While HIV-infected individuals with lymphogranuloma venereum should receive the same treatment regimens recommended for other patients, there is some evidence that HIV-infected patients may require more prolonged therapy and resolution may be delayed.
Chlamydia psittaci Infections
Tetracyclines are the drugs of choice for the treatment of C. psittaci infections (psittacosis); erythromycin or chloramphenicol is recommended for the treatment of psittacosis when tetracyclines are contraindicated. The CDC states that most individuals with psittacosis respond to an oral regimen of doxycycline or tetracycline hydrochloride. A regimen of IV doxycycline hyclate may be indicated for initial treatment of severely ill patients. Remission of symptoms usually is evident within 48-72 hours, but treatment should be continued for at least 10-14 days after fever abates since relapse can occur.
Nongonococcal Urethritis
While C. trachomatis is a frequent cause of nongonococcal urethritis, these infections can be caused by Ureaplasma urealyticum or Mycoplasma genitalium; Trichomonas vaginalis and herpes simplex virus (HSV) also are possible causes of nongonococcal urethritis. The CDC currently considers a single oral dose of azithromycin or a 7-day regimen of oral doxycycline the regimens of choice for the treatment of nongonococcal urethritis. Alternative regimens recommended by the CDC are a 7-day regimen of oral erythromycin base or ethylsuccinate or a 7-day regimen of oral ofloxacin or levofloxacin. Patients with persistent or recurrent urethritis who were not compliant with the treatment regimen or were reexposed to untreated sexual partner(s) should be retreated with the initial regimen. If the patient has recurrent and persistent urethritis, was compliant with the anti-infective regimen, and reexposure can be excluded, the CDC recommends retreatment with a 7-day regimen of oral erythromycin base or ethylsuccinate given in conjunction with a single 2-g dose of oral metronidazole.
Granuloma Inguinale (Donovanosis)
Tetracyclines are drugs of choice for the treatment of granuloma inguinale (donovanosis), caused by Calymmatobacterium granulomatis. The CDC recommends that donovanosis be treated with a regimen of oral doxycycline or oral co-trimoxazole or, alternatively, a regimen of oral ciprofloxacin, oral erythromycin, or oral azithromycin. Anti-infective treatment of donovanosis should be continued until all lesions have healed completely; a minimum of 3 weeks of treatment usually is necessary. If lesions do not respond within the first few days of therapy, the CDC recommends that a parenteral aminoglycoside (e.g., 1 mg/kg of gentamicin IV every 8 hours) be added to the regimen. Erythromycin is recommended for the treatment of donovanosis in pregnant and lactating women; addition of a parenteral aminoglycoside (e.g., gentamicin) to the regimen should be strongly considered in these women. Anti-infective treatment appears to halt progressive destruction of tissue, although prolonged duration of therapy often is required to enable granulation and re-epithelization of ulcers. Despite effective anti-infective therapy, donovanosis may relapse 6-18 months later. Individuals with HIV infection should receive the same treatment regimens recommended for other individuals with donovanosis; however, the CDC suggests that addition of a parenteral aminoglycoside to the regimen should be strongly considered in HIV-infected patients.
Pelvic Inflammatory Disease
Doxycycline is used in conjunction with other anti-infectives for the treatment of acute pelvic inflammatory disease (PID). One parenteral regimen recommended by the CDC and other clinicians for the treatment of PID in adults and adolescents is a 2-drug regimen of IV cefoxitin or IV cefotetan given in conjunction with oral or IV doxycycline. The parenteral regimen may be discontinued 24 hours after there is clinical improvement and oral doxycycline is then continued to complete 14 days of therapy. Because oral and IV doxycycline have similar bioavailabilities, the CDC states that either may be used for the initial phase of treatment. When tubo-ovarian abscess is present, some clinicians use clindamycin or metronidazole in addition to oral doxycycline to complete 14 days of therapy since this provides more effective anaerobic coverage. In another parenteral regimen recommended by the CDC for the treatment of PID, an initial regimen of IV clindamycin and IV or IM gentamicin is given. The parenteral regimen is discontinued 24 hours after there is clinical improvement and oral doxycycline is used to complete 14 days of therapy; however, if tubo-ovarian abscess is present, some clinicians substitute oral clindamycin instead of oral doxycycline for follow-up after the initial parenteral regimen. An alternative parenteral regimen recommended by the CDC for the treatment of PID is IV ampicillin and sulbactam given in conjunction with oral or IV doxycycline; this regimen has good coverage against C. trachomatis, N. gonorrhoeae, and anaerobes and is effective for patients with tubo-ovarian abscess. When an oral regimen is used for the treatment of PID in adults or adolescents, the CDC and many clinicians recommend use of a single IM dose of ceftriaxone, cefoxitin (with oral probenecid), or an equivalent second or third generation cephalosporin (e.g., ceftizoxime, cefotaxime) followed by a 14-day regimen of oral doxycycline with or without a 14-day regimen of oral metronidazole. The addition of metronidazole to this regimen provides coverage against bacterial vaginosis, which is frequently associated with PID. For further information on the treatment of PID.
Gram-negative Bacterial Infections
Tetracyclines are the drugs of first or second choice for the treatment of many infections caused by uncommon gram-negative bacteria.
Bartonella Infections
Doxycycline is considered an alternative agent for the treatment of infections caused by Bartonella quintana (formerly Rochalimaea quintana). B. quintana, a gram-negative bacilli, can cause cutaneous bacillary angiomatosis, trench fever, bacteremia, endocarditis, and chronic lymphadenopathy. B. quintana infections have been reported most frequently in immunocompromised patients (e.g., individuals with HIV infection), homeless individuals in urban areas, and chronic alcohol abusers. Optimum anti-infective regimens for the treatment of infections caused by B. quintana have not been identified, and various drugs have been used to treat these infections, including doxycycline, erythromycin, azithromycin, chloramphenicol, or cephalosporins. There is evidence that these infections tend to persist or recur and prolonged therapy (several months or longer) usually is necessary. Doxycycline has been used in the treatment of infections caused by Bartonella henselae (formerly Rochalimaea henselae) (e.g., cat scratch disease, bacillary angiomatosis, peliosis hepatitis); however, the possible role of tetracyclines in the treatment of these infections has not been determined. Cat scratch disease generally is a self-limited illness in immunocompetent individuals and may resolve spontaneously in 2-4 months; however, some clinicians suggest that anti-infective therapy be considered for acutely or severely ill patients with systemic symptoms, particularly those with hepatosplenomegaly or painful lymphadenopathy, and probably is indicated in immunocompromised patients. Anti-infectives also are indicated in patients with B. henselae infections who develop bacillary angiomatosis, neuroretinitis, or Parinaud’s oculoglandular syndrome. While the optimum anti-infective regimen for the treatment of cat scratch disease or other B. henselae infections has not been identified, some clinicians recommend use of erythromycin, azithromycin, doxycycline, ciprofloxacin, rifampin, co-trimoxazole, gentamicin, or third generation cephalosporins. HIV-infected individuals (especially severely immunosuppressed individuals) are at unusually high risk for severe disease caused by Bartonella and relapse or reinfection sometimes occurs following initial treatment of these infections. Therefore, although data are insufficient to make firm recommendations, the Prevention of Opportunistic Infections Working Group of the US Public Health Service and the Infectious Diseases Society of America (USPHS/IDSA) suggests that long-term suppression with erythromycin or doxycycline be considered to prevent recurrence of Bartonella infection in HIV-infected patients.
Brucellosis
Tetracyclines (doxycycline, tetracycline hydrochloride) generally are considered the drugs of choice for brucellosis. Limited data suggest that combined anti-infective therapy may reduce the likelihood of disease relapse, and some clinicians recommend that another anti-infective (e.g., streptomycin or gentamicin and/or rifampin) be used in conjunction with a tetracycline for the treatment of brucellosis. For treatment of serious brucellosis or when there are complications, including endocarditis, meningitis, or osteomyelitis, some clinicians recommend that an aminoglycoside (streptomycin or gentamicin) be used concomitantly with the tetracycline for the first 7-14 days of therapy; rifampin can also be used in the regimen to reduce the risk of relapse. Some experts recommend a 3-drug regimen that includes a tetracycline, an aminoglycoside, and rifampin for the treatment of brucellosis in patients with meningoencephalitis or endocarditis. Although data are limited, alternative regimens that have been suggested for the treatment of brucellosis include co-trimoxazole with or without gentamicin or rifampin (recommended for use in children when tetracyclines are contraindicated); ciprofloxacin (or ofloxacin) and rifampin; and chloramphenicol with or without streptomycin. Postexposure prophylaxis with anti-infectives is not generally recommended after possible exposure to endemic brucellosis; however, use of an anti-infective regimen recommended for the treatment of brucellosis (e.g., doxycycline and rifampin) should be considered following a high-risk exposure to Brucella. These high-risk exposures include needle-stick injuries involving the brucella vaccine available for veterinary use (a brucella vaccine for use in humans is not available); inadvertent laboratory exposure to the organism; or confirmed exposure in the context of biologic warfare or bioterrorism.
Burkholderia Infections
A tetracyclines used in conjunction with streptomycin is considered a regimen of choice for the treatment of glanders caused by B. mallei (formerly Ps. mallei). Although other anti-infectives (e.g., imipenem, ceftazidime) are considered drugs of choice for the treatment of melioidosis caused by susceptible Burkholderia pseudomallei (formerly Pseudomonas pseudomallei), some clinicians suggest that a regimen of chloramphenicol, doxycycline, and co-trimoxazole is one of several alternative regimens.
Gonorrhea and Associated Infections
Some manufacturers state that oral doxycycline or oral tetracycline hydrochloride can be used as alternatives for the treatment of uncomplicated gonorrhea. However, tetracyclines are considered inadequate therapy for the treatment of gonorrhea and are not recommended by the CDC for the treatment of uncomplicated or disseminated gonorrhea. The CDC and many clinicians currently recommend use of tetracyclines for presumptive treatment of coexisting chlamydial infections in patients being treated for gonococcal infections. For the treatment of epididymitis most likely caused by gonococcal or chlamydial infection, the CDC recommends a single 250-mg IM dose of ceftriaxone given in conjunction with a 10-day regimen of oral doxycycline (100 mg twice daily). For epididymitis most likely to be caused by enteric bacteria (e.g., Escherichia coli), for those allergic to tetracyclines and/or cephalosporins, or for patients older than 35 years of age, the CDC recommends a 10-day regimen of oral ofloxacin or oral levofloxacin. Empiric treatment of epididymitis is indicated before in vitro culture results are available. As an adjunct to therapy, bed rest, scrotal elevation, and analgesics are recommended until fever and local inflammation have subsided. Although most patients can be treated as outpatients, hospitalization should be considered when severe pain suggests other diagnoses (e.g., torsion, testicular infarction, abscess) or when patients are febrile or might be noncompliant. For the treatment of proctitis likely to be caused by N. gonorrhoeae or C. trachomatis, the CDC recommends a single 125-mg IM dose of ceftriaxone (or another anti-infective effective against rectal and genital gonorrhea) given in conjunction with a 7-day regimen of oral doxycycline (100 mg twice daily).
Plague
Tetracyclines (doxycycline, tetracycline) are used for the treatment of plague caused by Yersinia pestis. Streptomycin (or gentamicin) with or without a tetracycline generally is considered the drug of choice for the treatment of plague. Alternative drugs recommended when aminoglycosides are not used include doxycycline (or tetracycline), chloramphenicol, or co-trimoxazole (may be less effective than other alternatives). Based on results of in vitro and animal testing, ciprofloxacin (or other fluoroquinolones) also is recommended as an alternative for the treatment of plague. Chloramphenicol generally is considered the drug of choice for the treatment of plague meningitis. Anti-infective regimens recommended for the treatment of naturally occurring or endemic bubonic, septicemic, or pneumonic plague also are recommended for the treatment of plague that occurs following exposure to Y. pestis in the context of biologic warfare or bioterrorism. These exposures would most likely result in primary pneumonic plague. Prompt initiation of anti-infective therapy (within 18-24 hours of onset of symptoms) is essential in the treatment of pneumonic plague. Some experts (e.g., the US Working Group on Civilian Biodefense, US Army Medical Research Institute of Infectious Diseases) recommend that treatment of plague in the context of biologic warfare or bioterrorism should be initiated with a parenteral anti-infective regimen of streptomycin (or gentamicin) or, alternatively, doxycycline, ciprofloxacin, or chloramphenicol, although an oral regimen (doxycycline, ciprofloxacin) may be substituted when the patient’s condition improves or if parenteral therapy is unavailable. Postexposure prophylaxis with anti-infectives is recommended after high-risk exposures to plague, including close exposure to individuals with naturally occurring plague or laboratory exposure to viable Y. pestis. In the context of biologic warfare or bioterrorism, some experts (e.g., the US Working Group on Civilian Biodefense, US Army Medical Research Institute of Infectious Diseases) recommend that asymptomatic individuals with exposure to plague aerosol or asymptomatic individuals with household, hospital, or other close contact (within about 2 m) with an individual who has pneumonic plague should receive postexposure anti-infective prophylaxis; however, any exposed individual who develops a temperature of 38.5°C or higher or new cough should promptly receive a parenteral anti-infective for treatment of the disease. An oral regimen of doxycycline or ciprofloxacin usually is recommended for such prophylaxis. Alternatives suggested for postexposure prophylaxis include oral tetracycline, co-trimoxazole, or oral chloramphenicol (an oral preparation is not commercially available in the US). Although plague vaccine (no longer commercially available in the US) was previously recommended to provide protection against Y. pestis infection, the vaccine was effective for preventing or ameliorating bubonic plague but was not effective for prophylaxis against exposure to aerosolized Y. pestis and therefore did not provide protection against pneumonic plague.
Tularemia
Tetracyclines (usually doxycycline) are used as alternative agents for the treatment of tularemia caused by Francisella tularensis. Streptomycin (or gentamicin) generally are considered the drugs of choice for this infection. Alternatives for the treatment of tularemia include tetracyclines (doxycycline), chloramphenicol, or ciprofloxacin. Anti-infective regimens recommended for the treatment of naturally occurring or endemic tularemia also are recommended for the treatment of tularemia that occurs following exposure to F. tularensis in the context of biologic warfare or bioterrorism. However, the fact that a fully virulent streptomycin-resistant strain of F. tularensis was developed in the past for use in biologic warfare should be considered. Exposures to F. tularensis in the context of biologic warfare or bioterrorism would most likely result in inhalational tularemia with pleuropneumonitis, although the organism also can infect humans through the skin, mucous membranes, and GI tract. Postexposure prophylaxis with anti-infectives usually is not recommended after possible exposure to natural or endemic tularemia (e.g., tick bite, rabbit or other animal exposure) and is unnecessary in close contacts of tularemia patients since human-to-human transmission of the disease is not known to occur. However, postexposure prophylaxis is recommended following a high-risk laboratory exposure to F. tularensis (e.g., spill, centrifuge accident, needlestick injury). In the context of biologic warfare or bioterrorism, some experts (e.g., the US Working Group on Civilian Biodefense, US Army Medical Research Institute of Infectious Diseases) recommend that asymptomatic individuals with exposure to F. tularensis receive postexposure anti-infective prophylaxis; however, any individual who develops an otherwise unexplained fever or flu-like illness within 14 days of presumed exposure should promptly receive a parenteral anti-infective for treatment of the disease. Oral doxycycline (or oral tetracycline) or oral ciprofloxacin usually is recommended for postexposure prophylaxis following such exposures.
Other Gram-negative Bacterial Infections
When the drugs of choice are contraindicated or are ineffective, tetracyclines are used as alternatives to erythromycins, ceftriaxone, or co-trimoxazole in the treatment of Campylobacter fetus infections. Tetracyclines are used as alternatives to penicillin G in the treatment of infections caused by Leptotrichia buccalis (Vincent’s infection). Although doxycycline has been used for the treatment of chancroid caused by Haemophilus ducreyi, tetracyclines are not included in current CDC recommendations for the treatment of chancroid. Tetracyclines have been effective in the treatment of rat-bite fever caused by Spirillum minus and Haverhill fever caused by Streptobacillus moniliformis; however, penicillin G generally is preferred for the treatment of these infections. Tetracyclines are considered alternatives to penicillin G for the treatment of infections caused by Pasteurella multocida. Some clinicians recommend minocycline as an alternative to co-trimoxazole for the treatment of infections caused by Stenotrophomonas maltophilia. Tetracyclines, with or without rifampin, have been used in the treatment of Legionnaires’ disease caused by Legionella pneumophila. Macrolides or fluoroquinolones generally are considered the drugs of choice for the treatment of pneumonia caused by L. pneumophila and doxycycline and co-trimoxazole are alternatives. A parenteral regimen usually is necessary for the initial treatment of severe Legionnaires’ disease and the addition of oral rifampin is recommended during the first 3-5 days of macrolide or doxycycline therapy in severely ill and/or immunocompromised patients; after a response is obtained, rifampin can be discontinued and therapy changed to an oral regimen. Although minocycline has been used to eliminate meningococci from the nasopharynx of asymptomatic N. meningitidis carriers in situations in which the risk of meningococcal meningitis is high, adverse CNS effects (e.g., vestibular symptoms) are reported occasionally with minocycline and the CDC and AAP currently recommend other anti-infective agents (i.e., rifampin, ceftriaxone, ciprofloxacin) for the treatment of carriers of N. meningitidis. Minocycline is not indicated for the treatment of infections caused by N. meningitidis. Tetracyclines have been used for the treatment of infections caused by susceptible Acinetobacter, Bacteroides, Enterobacter aerogenes, Escherichia coli, and Shigella; respiratory tract infections caused by H. influenzae; and respiratory tract and urinary tract infections caused by K. pneumoniae. However, many strains of these gram-negative bacteria have been shown to be resistant to tetracyclines and the drugs generally should not be used empirically in infections suspected to be caused by these organisms. Tetracyclines should be used in the treatment of infections caused by common gram-negative bacteria only when other appropriate anti-infectives (e.g., aminoglycosides) are contraindicated or are ineffective and when results of in vitro susceptibility tests indicate that the organisms are susceptible.
Gram-positive Bacterial Infections
Anthrax
Doxycycline and tetracycline hydrochloride are used in the treatment of anthrax and for postexposure prophylaxis following a suspected or confirmed exposure to aerosolized anthrax spores (inhalational anthrax). Doxycycline is the preferred tetracycline for inhalational anthrax based on efficacy data in monkey studies and ease of administration. Parenteral penicillin generally has been considered the drug of choice for the treatment of naturally occurring or endemic anthrax caused by susceptible strains of Bacillus anthracis, including clinically apparent inhalational, GI, or meningeal anthrax and anthrax septicemia, although IV ciprofloxacin or IV doxycycline also are recommended. However, B. anthracis strains with natural resistance to penicillins have been reported and there are published reports of B. anthracis strains that have been engineered to have tetracycline and penicillin resistance as well as resistance to other anti-infectives (e.g., macrolides, chloramphenicol, rifampin). Therefore, it has been postulated that exposures to B. anthracis that occur in the context of biologic warfare or bioterrorism may involve bioengineered resistant strains and this concern should be considered when selecting initial therapy for treatment of anthrax that occurs as the result of bioterrorism-related exposures or when selecting anti-infective agents for postexposure prophylaxis following such exposures. For additional information on treatment of anthrax and recommendations for postexposure prophylaxis following exposure to anthrax spores.
Postexposure Prophylaxis
Doxycycline is used for inhalational anthrax (postexposure) to reduce the incidence or progression of disease following suspected or confirmed exposure to aerosolized B. anthracis spores. Ciprofloxacin or doxycycline are considered initial drugs of choice for postexposure prophylaxis following exposure to aerosolized anthrax spores that occurs in the context of biologic warfare or bioterrorism. There is no evidence to date that ciprofloxacin is more or less effective than doxycycline for such postexposure prophylaxis. During the bioterrorism-related exposures to B. anthracis spores during September and October 2001, the CDC initially recommended postexposure prophylaxis with either ciprofloxacin or doxycycline, but later revised these recommendations because of the large number of exposed individuals. At that time, the CDC suggested that use of doxycycline for postexposure prophylaxis was preferable since widespread use of any anti-infective agent can promote resistance to that drug and because many common pathogens already are resistant to tetracycline but still susceptible to fluoroquinolone and this tactic might preserve effectiveness of ciprofloxacin against these common pathogens. The US Working Group on Civilian Biodefense currently recommends use of ciprofloxacin as the initial drug of choice for postexposure prophylaxis and recommend use of doxycycline as an alternative if the organism is found to be susceptible. These experts also state that in vitro studies suggest that oral tetracycline hydrochloride could be substituted for doxycycline, if necessary. Ultimately, selection of an anti-infective agent for postexposure prophylaxis should be based on the clinical setting, susceptibility of the strain involved, and reported adverse effects associated with the drugs and either doxycycline or ciprofloxacin may be preferable for an individual patient. Anti-infective prophylaxis should be continued until exposure to B. anthracis has been excluded. If exposure is confirmed, postexposure vaccination with anthrax vaccine (if available) may be indicated in conjunction with prophylaxis. Because of the possible persistence of anthrax spores in lung tissue following an aerosol exposure, the CDC and other experts recommend that postexposure prophylaxis be continued for 60 days after exposure. Because of potential adverse effects from prolonged use of doxycycline in infants and children, amoxicillin is an alternative to complete the postexposure prophylaxis regimen when susceptibility to penicillin is known. The CDC states that anti-infective prophylaxis is not necessary for workers in contaminated environments who wear appropriate personal protective equipment and who have received the complete vaccine series, unless a breach of respiratory protection occurs. However, remediation workers involved in clean up and decontamination of B. anthracis-contaminated sites who have not been vaccinated with the complete 6-dose recommended regimen of anthrax vaccine should receive anti-infective prophylaxis, regardless of other methods being used to protect these individuals from exposure. This recommendation also applies to workers entering areas that already have been remediated but have not yet been cleared for general occupancy. Unvaccinated or incompletely vaccinated remediation workers should begin anti-infective prophylaxis at the time of first entry into the contaminated area, and such prophylaxis should be continued until at least 60 days after last entry into the area for unvaccinated workers. Remediation workers who have received all or part of the 6-dose vaccine regimen should continue anti-infective prophylaxis for at least 30 days and should complete the vaccination regimen. In addition, it might be prudent to continue anti-infective prophylaxis until 7-14 days after the third vaccine dose is administered. Remediation workers with repeated entries into contaminated sites over a prolonged period of time could require anti-infective prophylaxis for considerably longer than the 60 days recommended for individuals with a single exposure. To date, some remediation workers have received anti-infective prophylaxis for more than 6 months. If anthrax vaccine is administered to an individual while their risk of exposure to anthrax spores continues, the CDC recommends concomitant anti-infective prophylaxis throughout the period of risk and for 60 days after the risk of exposure has ended, unless the 6-dose series of anthrax vaccine has been completed and annual boosters are up-to-date. Although controlled studies evaluating doxycycline for aerosolized anthrax exposure have not been conducted for ethical reasons, the drug has been evaluated in a rhesus monkey model of inhalational anthrax. In this study, mortality due to anthrax was significantly lower in monkeys that received doxycycline compared with those that received placebo. Peak serum concentrations of doxycycline associated with survival in this rhesus monkey model were within the range usually observed with usual dosages of the drug.
Treatment of Inhalational Anthrax
The rapid course of symptomatic inhalational anthrax and high mortality rate make early initiation of anti-infective therapy essential. While monotherapy with IV penicillin G, ciprofloxacin, or doxycycline has been recommended for the treatment of anthrax that occurs as the result of natural or endemic exposures, the CDC and other experts (e.g., US Working Group on Civilian Biodefense) recommend that treatment of clinically apparent inhalational anthrax that occurs as the result of exposure to anthrax spores in the context of biologic warfare or bioterrorism should be initiated with a multiple-drug parenteral regimen that includes ciprofloxacin or doxycycline and 1 or 2 other anti-infectives predicted to be effective. Drugs to be included in the initial treatment regimen with ciprofloxacin or doxycycline should be selected based on in vitro susceptibility, possibility of efficacy, adverse effects, and cost. Based on in vitro data, drugs that have been suggested as possibilities to augment ciprofloxacin and doxycycline in such multiple-drug regimens include chloramphenicol, clindamycin, rifampin, vancomycin, clarithromycin, imipenem, penicillin, or ampicillin. If meningitis is established or suspected, some clinicians suggest a multiple-drug regimen that includes ciprofloxacin (rather than doxycycline) and chloramphenicol, rifampin, or penicillin. Because of the difficulty in making a rapid microbiologic diagnosis of anthrax, high-risk individuals who develop fever or other evidence of systemic infection should promptly receive therapy for possible anthrax infection while waiting for results of laboratory studies. If large numbers of individuals require treatment in mass casualty settings, IV therapy with a multiple-drug parenteral regimen may not be possible. In these circumstances, oral therapy with a regimen recommended for postexposure prophylaxis of inhalational anthrax is an option. Because of the possible persistence of anthrax spores in lung tissue, anti-infective therapy for the treatment of inhalational anthrax that occurs as the result of exposure to aerosolized spores in the context of biologic warfare or bioterrorism should be continued for 60 days. Oral anti-infective therapy can be substituted for IV therapy as soon as the patient’s clinical condition improves. Although tetracyclines are not usually used in children younger than 8 years of age, doxycycline can be used in infants and children for the initial treatment of anthrax if considered necessary.If infants and children with inhalational anthrax have clinical improvement while receiving the initial parenteral regimen, an oral regimen of 1 or 2 anti-infectives (including either doxycycline or ciprofloxacin) may be used to complete the first 14-21 days of therapy. Because of potential adverse effects from prolonged use of doxycycline in infants and children, amoxicillin is an option for completion of the remaining 60 days of therapy but is not recommended for initial therapy. For the treatment of inhalational anthrax in pregnant women, the benefits of doxycycline therapy outweigh the risks and the CDC and other experts (e.g., US Working Group on Civilian Biodefense) state that doxycycline can be used when necessary for the treatment of inhalational anthrax. Recommendations for the treatment of anthrax in immunocompromised patients are the same as those for patients who are immunocompetent.
Treatment of Cutaneous Anthrax
Natural penicillins (e.g., oral penicillin V, IM penicillin G benzathine, IM penicillin G procaine) generally have been considered drugs of choice for the treatment of mild, uncomplicated cutaneous anthrax caused by susceptible strains of B. anthracis that occurs as the result of naturally occurring or endemic exposure to anthrax, although some clinicians suggest use of oral fluoroquinolones (ciprofloxacin, ofloxacin, levofloxacin), oral amoxicillin, or oral doxycycline if in vitro tests indicate susceptibility. For the treatment of cutaneous anthrax that occurs following exposure to B. anthracis spores in the context of biologic warfare or bioterrorism, the CDC and other experts (e.g., US Working Group on Civilian Biodefense) recommend use of oral ciprofloxacin or oral doxycycline for initial therapy. Therapy may be changed to oral amoxicillin if results of in vitro testing indicate that the organism is susceptible to the drug and the patient is improving. Recommendations for treatment of cutaneous anthrax in immunocompromised patients are the same as those for patients who are immunocompetent. Use of a multiple-drug parenteral anti-infective regimen is recommended for the initial treatment of cutaneous anthrax when there are signs of systemic involvement, extensive edema, or lesions on the head and neck. Whether infants and young children are at increased risk for systemic dissemination of cutaneous anthrax infections is not known; however, a child 7 months of age infected following a bioterrorism-related exposure developed systemic illness after onset of cutaneous anthrax. Therefore, the CDC recommends that a parenteral regimen be used for the initial treatment of cutaneous anthrax in children younger than 2 years of age and use of a combination regimen should be considered. If a parenteral regimen is indicated for the treatment of cutaneous anthrax and if infants and children have clinical improvement while receiving the parenteral regimen, an oral regimen of 1 or 2 anti-infectives (including either doxycycline or ciprofloxacin) may be used to complete the first 7-10 days of therapy. Because of potential adverse effects from prolonged use of doxycycline in infants and children, amoxicillin is an option for completion of the remaining 60 days of therapy, but is not recommended for initial therapy. Although 5-10 days of anti-infective therapy usually is recommended for the treatment of mild, uncomplicated cutaneous anthrax that occurs as the result of natural or endemic exposures to anthrax, the CDC and other experts recommend that therapy be continued for 60 days if the cutaneous infection occurred as the result of exposure to aerosolized anthrax spores since the possibility of inhalational anthrax would also exist. Anti-infective therapy may limit the size of the cutaneous anthrax lesion and it usually becomes sterile within the first 24 hours of treatment, but the lesion will still progress through the black eschar stage despite effective treatment.
Other Gram-positive Bacterial Infections
Tetracyclines are used as alternatives to penicillin G or metronidazole for the treatment of Clostridium tetani infections. When penicillin G is ineffective or is contraindicated, tetracyclines are used in the treatment of actinomycosis caused by Actinomyces israelii. Some clinicians recommend that long-term tetracycline hydrochloride therapy be used as follow-up treatment after penicillin G in severe cases of the disease. Tetracyclines are considered alternatives to co-trimoxazole for the treatment of nocardiosis. Some clinicians recommend a regimen of a sulfonamide and either minocycline or amikacin as an alternative to co-trimoxazole; although doxycycline or minocycline are also recommended alone as alternative regimens for the treatment of nocardiosis. Because of increasing resistance, tetracyclines are not considered drugs of choice for infections caused by gram-positive cocci (e.g., Staphylococcus or Streptococcus and should not be used empirically in infections suspected to be caused by these organisms. Tetracyclines should be used in the treatment of infections caused by Staphylococcus or Streptococcus only when other appropriate anti-infectives (e.g., penicillins, cephalosporins, erythromycin, clindamycin, vancomycin) are ineffective or are contraindicated and when results of in vitro susceptibility tests indicate that the organisms are susceptible to the drugs. If tetracyclines are used in infections caused by b-hemolytic streptococci, therapy should be continued for at least 10 days.
Acne
Tetracyclines are used orally in the treatment of moderate to severe inflammatory acne vulgaris. The drugs are not indicated in the treatment of noninflammatory acne. Therapy of acne vulgaris must be individualized and frequently modified depending on the types of acne lesions that predominate and the response to therapy. Oral minocycline may be effective in patients with inflammatory acne unresponsive to oral tetracycline hydrochloride or oral erythromycin. Although it has been suggested that failure to respond to anti-infective therapy may be caused by the development of resistance by P. acnes to the drug being administered, resistance is rare in this organism and failure to respond to anti-infective therapy appears to be more frequently caused by other factors (e.g., poor patient compliance, emotional or psychological factors, use of comedogenic cosmetic products, the presence of deep nodular or cystic lesions, sinus tract formation). Preliminary studies using topical clindamycin, topical erythromycin, and topical tetracycline hydrochloride (a topical solution is no longer commercially available in the US) indicate that topical anti-infectives are as effective as oral tetracycline hydrochloride or oral erythromycin in the treatment of mild to moderate inflammatory acne. Some clinicians recommend that oral anti-infective therapy be used initially in the treatment of moderate to severe inflammatory acne vulgaris since the response to topical therapy may be delayed. A topical anti-infective is then used concomitantly after a few weeks, and the oral anti-infective is slowly discontinued. However, further controlled studies are needed to determine when each route of administration is preferred and when combined topical and oral administration of anti-infectives is indicated in the treatment of inflammatory acne vulgaris.
Respiratory Tract Infections
Tetracyclines are used in the treatment of respiratory tract infections caused by M. pneumoniae, Haemophilus influenzae, Klebsiella, and Streptococcus pneumoniae. Tetracyclines are used in the treatment of atypical pneumonia caused by Mycoplasma pneumoniae. Available data suggest that erythromycin and tetracycline are equally effective in shortening the duration of clinical symptoms and hastening radiographic improvement in adults with mycoplasma pneumonia, despite failure to eradicate the pathogen from nasopharyngeal or sputum cultures. Although conflicting data regarding the efficacy of antibiotic therapy of mycoplasma pneumonia in children have been reported, some clinicians suggest that erythromycin is preferred for treating children with the disease. The optimal duration of antibiotic therapy for mycoplasma pneumonia has not been established; however, because of the persistence of the pathogen, some clinicians recommend that such therapy be continued for 2-4 weeks to minimize the possibility of relapse.
Community-acquired Pneumonia
Tetracyclines are used in the treatment of community-acquired pneumonia (CAP). Initial treatment of CAP generally involves use of an empiric anti-infective regimen based on the most likely pathogens; therapy may then be changed (if possible) to a pathogen-specific regimen based on results of in vitro culture and susceptibility testing, especially in hospitalized patients. The most appropriate empiric regimen varies depending on the severity of illness at the time of presentation and whether outpatient treatment or hospitalization in or out of an intensive care unit (ICU) is indicated and the presence or absence of cardiopulmonary disease and other modifying factors that increase the risk of certain pathogens (e.g., penicillin- or multidrug-resistant S. pneumoniae, enteric gram-negative bacilli, Ps. aeruginosa). For both outpatients and inpatients, most experts recommend that an empiric regimen for the treatment of CAP include an anti-infective active against S. pneumoniae since this organism is the most commonly identified cause of bacterial pneumonia and causes more severe disease than many other common CAP pathogens. When used in empiric regimens for the treatment of CAP, tetracyclines provide coverage against C. pneumoniae, M. pneumoniae, Haemophilus influenzae, and Legionella. Although tetracyclines may also provide some coverage for S. pneumoniae, many isolates of this organisms are resistant to tetracyclines. Doxycycline generally is the tetracycline recommended for empiric treatment of CAP because of good oral bioavailability, convenient twice-daily regimen, and tolerability. In most situations, either doxycycline or a macrolide is included in outpatient empiric CAP regimens. Although the IDSA doesn’t make a distinction between macrolides and doxycycline, the ATS states that use of a macrolide is preferred (rather than doxycycline) because S. pneumoniae may be resistant to tetracyclines and recommends use of doxycycline as an alternative in patients hypersensitive or intolerant of macrolides. Tetracyclines are not usually recommended for empiric CAP regimens in patients with severe infections admitted to an intensive care unit (ICU), but doxycycline may be used as an alternative to macrolides in inpatient CAP regimens in patients hospitalized in a non-ICU setting. The duration of CAP therapy depends on the causative pathogen, illness severity at the onset of anti-infective therapy, response to treatment, comorbid illness, and complications. CAP secondary to S. pneumoniae generally can be treated for 7-10 days or 72 hours after the patient becomes afebrile. CAP caused by bacteria that can necrose pulmonary parenchyma generally should be treated for at least 2 weeks. Patients chronically treated with corticosteroids also may require at least 2 weeks of therapy. CAP caused by M. pneumoniae or C. pneumoniae probably should be treated for at least 10-14 days. CAP caused by Legionella in immunocompetent patients also probably should be treated for at least 10-14 days, although some clinicians recommend 21 days.
Outpatient
Regimens for CAP Pathogens most frequently involved in outpatient CAP include S. pneumoniae, M. pneumoniae, C. pneumoniae, respiratory viruses, and H. influenzae (especially in cigarette smokers). Therefore, for empiric outpatient treatment of acute CAP in immunocompetent adults, the IDSA recommends monotherapy with an oral macrolide (azithromycin, clarithromycin, erythromycin), oral doxycycline, or an oral fluoroquinolone active against S. pneumoniae (e.g., gatifloxacin, levofloxacin, moxifloxacin). Some experts prefer macrolides or doxycycline in patients younger than 50 years of age who have no comorbidities and fluoroquinolones for other individuals. The IDSA states that alternative empiric outpatient regimens include oral amoxicillin and clavulanate or certain oral cephalosporins (cefpodoxime, cefprozil, cefuroxime axetil). For outpatient treatment of CAP in immunocompetent adults without cardiopulmonary disease or other modifying factors that would increase the risk of multidrug-resistant S. pneumoniae or gram-negative bacteria, the American Thoracic Society (ATS) recommends an empiric regimen of monotherapy with azithromycin or clarithromycin or, alternatively, doxycycline. However, for the outpatient treatment of immunocompetent adults with cardiopulmonary disease (congestive heart failure or chronic obstructive pulmonary disease [COPD]) and/or other modifying factors that increase the risk for multidrug-resistant S. pneumoniae or gram-negative bacteria, the ATS recommends a 2-drug empiric regimen consisting of a b-lactam anti-infective (e.g. oral cefpodoxime, oral cefuroxime axetil, high-dose amoxicillin, amoxicillin and clavulanate, parenteral ceftriaxone followed by oral cefpodoxime) and a macrolide or doxycycline or, alternatively, monotherapy with a fluoroquinolone active against S. pneumoniae (e.g., ciprofloxacin, ofloxacin, gatifloxacin, levofloxacin, moxifloxacin, sparfloxacin, trovafloxacin [risk of hepatic toxicity should be considered]). The CDC suggests that use of these oral fluoroquinolones in the outpatient treatment of CAP be reserved for when other anti-infectives are ineffective or cannot be used or when highly penicillin-resistant S. pneumoniae (i.e., penicillin MICs 4 mcg/mL or greater) are identified as the cause of infection.
Inpatient Regimens for CAP
In addition to S. pneumoniae, other pathogens often involved in inpatient CAP are H. influenzae, enteric gram-negative bacilli, S. aureus, Legionella, M. pneumoniae, C. pneumoniae, and viruses. Patients with severe CAP admitted into the ICU may have Ps. aeruginosa infections (especially those with underlying bronchiectasis or cystic fibrosis) and Enterobacteriaceae often are involved. In addition, anaerobic infection should be suspected in patients with aspiration pneumonia or lung abscess. Inpatient treatment of CAP is initiated with a parenteral regimen, although therapy may be changed to an oral regimen if the patient is improving clinically, is hemodynamically stable, and is able to ingest drugs. CAP patients usually have a clinical response within 3-5 days after initiation of therapy and failure to respond to the initial empiric regimen generally indicates an incorrect diagnosis, host failure, inappropriate anti-infective regimen (drug selection, dosage, route), unusual pathogen, adverse drug reaction, or complication (e.g., pulmonary superinfection, empyema). For empiric inpatient treatment of CAP in immunocompetent adults who require hospitalization in a non-ICU setting, the IDSA recommends a 2-drug regimen consisting of a parenteral b-lactam anti-infective (e.g., cefotaxime, ceftriaxone, ampicillin and sulbactam, piperacillin and tazobactam) and a macrolide (e.g., azithromycin, clarithromycin, erythromycin) or monotherapy with a fluoroquinolone active against S. pneumoniae (e.g., gatifloxacin, levofloxacin, moxifloxacin). The IDSA does not recommend use of doxycycline for inpatient empiric regimens. For empiric inpatient treatment of CAP in immunocompetent adults who are hospitalized in a non-ICU setting and have cardiopulmonary disease (congestive heart failure or chronic obstructive pulmonary disease [COPD]) and/or other modifying factors that increase the risk for multidrug-resistant S. pneumoniae or gram-negative bacteria, the ATS recommends a 2-drug regimen consisting of a parenteral b-lactam anti-infective (cefotaxime, ceftriaxone, ampicillin and sulbactam, high-dose ampicillin) and an oral or IV macrolide (azithromycin or clarithromycin; doxycycline can be used in those with macrolide sensitivity or intolerance) or, alternatively, monotherapy with an IV fluoroquinolone active against S. pneumoniae. If anaerobes are documented or lung abscess is present, clindamycin or metronidazole should be added to the regimen. For CAP patients admitted to a non-ICU setting who do not have cardiopulmonary disease or other modifying factors, the ATS suggests an empiric regimen of monotherapy with IV azithromycin; for those with macrolide sensitivity or intolerance, a 2-drug regimen of doxycycline and a b-lactam or monotherapy with a fluoroquinolone active against S. pneumoniae can be used. For information on empiric regimens for the inpatient treatment of CAP in patients who require hospitalization in an ICU, see Inpatient Regimens for CAP under Respiratory Tract Infections: Community-acquired Pneumonia.
Spirochetal Infections
Syphilis
Tetracyclines (doxycycline, tetracycline hydrochloride) are used as alternative agents for the treatment of syphilis caused by Treponema pallidum. Parenteral penicillin G is the treatment of choice for all stages of syphilis. Although efficacy is not well documented, the CDC states that use of oral doxycycline or tetracycline hydrochloride can be considered to treat primary, secondary, latent, or tertiary syphilis (not neurosyphilis) in nonpregnant adults and adolescents hypersensitive to penicillin if compliance and follow-up serologic testing can be ensured. Although there is less clinical experience with doxycycline than tetracycline hydrochloride for the treatment of syphilis, compliance probably is better with doxycycline. If compliance and follow-up with nonpenicillin regimens cannot be ensured, patients with primary, secondary, latent, or tertiary syphilis who are hypersensitive to penicillin should be desensitized, if necessary, and treated with penicillin. Patients with neurosyphilis who are hypersensitive to penicillin should be desensitized, if necessary, and treated with penicillin or, alternatively, treated in consultation with the CDC or other clinicians who have expertise in the treatment of neurosyphilis. Although the AAP states that doxycycline or tetracycline hydrochloride can be used to treat primary, secondary, or latent syphilis (not tertiary or neurosyphilis) in children 8 years of age or older with penicillin hypersensitivity, the CDC states that infants and children with syphilis who are hypersensitive to penicillin should be desensitized, if necessary, and treated with penicillin. Tetracyclines should not be used to treat syphilis in pregnant women hypersensitive to penicillin. There are no proven alternatives to penicillin for the treatment of syphilis during pregnancy, and pregnant women with a history of penicillin hypersensitivity should be desensitized, if indicated, and treated with penicillin. There is little information on use of penicillin alternatives for the treatment of syphilis in HIV-infected patients. The CDC states that HIV-infected patients with primary or secondary syphilis should be managed according to the recommendations for HIV-negative, penicillin-hypersensitive patients. However, if compliance and follow-up cannot be assured, HIV-infected individuals with latent syphilis who are hypersensitive to penicillin should be desensitized and treated with penicillin. For information on current recommendations regarding treatment and follow-up for all stages and forms of syphilis.
Lyme Disease
Doxycycline is considered a drug of choice in the treatment of early Lyme disease. Lyme disease (Lyme borreliosis) is a spirochetal disease caused by Borrelia burgdorferi and currently is the most common tick-borne infection in the US, although the disease has a worldwide distribution. Lyme disease is transmitted in the US principally by the ticks Ixodes scapularis (also called I. dammini) and I. pacificus. Current evidence suggests that the tick usually must be attached for at least 24-48 hours to transmit the infection. For information on usual strategies for prevention of Lyme disease, including use of the vaccine. Lyme disease generally occurs in 3 stages that typically occur in sequence, with different clinical manifestations at each stage. However, the disease, like syphilis, can have a variable presentation, and in individual patients the 3 stages can occur alone or may overlap. The different stages of Lyme disease also have been classified in a manner analogous to that of syphilis, in which stages 1 and 2 represent early localized and early disseminated infection, respectively, and stage 3 represents late infection. Early localized (stage 1) Lyme disease, which may appear days to weeks after transfer of the spirochete to the human host, usually is manifested by a characteristic skin lesion, erythema migrans (erythema chronicum migrans). Current data suggest that erythema migrans develops in at least 80-90% of patients and typically begins as an erythematous macule or papule at the site of the tick bite that expands circularly to form a large (e.g., up to 70 cm in diameter) annular lesion, sometimes with partial central clearing. Some patients with Lyme disease develop secondary multiple skin lesions, which may resemble the primary lesion somewhat but generally are smaller and migrate less. Erythema migrans often is accompanied by fever, flu-like constitutional symptoms (e.g., chills, malaise, fatigue, headache), or regional lymphadenopathy. Early disseminated (stage 2) infection, which may be manifested days to months after the initial infection, is associated with hematogenous and lymphatic dissemination of the infection and characteristic symptoms in various organ systems, principally the skin, nervous system, musculoskeletal system, and/or heart. Severe headache and neck stiffness, generally transient, and meningitis with cranial (e.g., facial nerve [Bell’s] palsy) or peripheral (e.g., radiculoneuropathy of the limbs or trunk) neuropathy are nervous system manifestations that can occur in the early stages of the disease. Optic nerve involvement, which may occur as a result of inflammation or increased intracranial pressure, has been reported principally in children and may lead to blindness. Manifestations of acute neuroborreliosis may develop in about 15% of untreated patients within weeks after the period of early disseminated infection. Acute neurologic abnormalities typically resolve or improve within weeks or months even in untreated patients, although a small percentage (5%) of untreated patients may exhibit chronic neurologic manifestations. Symptoms of musculoskeletal involvement, which generally are variable and intermittent and occur in about 60% of untreated patients in the US, may include migratory pain in the joints, bursae, tendons, muscle, and bone and, rarely, a deep myositis; brief episodes of arthritis, principally involving one or only a few large joints (particularly the knee), also may occur during the early stages of the disease. Cardiac involvement appears to occur in approximately 4-10% of untreated patients with Lyme disease, usually within 1-3 months after infection. The most frequently occurring cardiac abnormality is AV block of varying degrees (first-degree, Wenckebach, or complete heart block), which is usually of short duration but potentially may require temporary insertion of a pacemaker. Persistent or late infection (stage 3), which occurs months to years following the tick bite, generally is manifested by intermittent episodes of arthritis, which may become chronic (defined as continuous joint inflammation for 1 year or longer) but eventually resolves even in untreated patients (i.e., the number of patients with recurrent episodes of arthritis decreases by approximately 10-20% each year). Some evidence suggests that chronic arthritis, which occurs in a small percentage of patients despite recommended anti-infective treatment for Lyme disease, may be related to certain immunogenetic or immune factors (e.g., presence of HLA-DR4 haplotype). Other manifestations of late Lyme disease include subtle central or peripheral neurologic abnormalities such as mild subacute encephalopathy (e.g., characterized by memory loss, behavioral changes, somnolence) and/or polyneuropathy (e.g., characterized by intermittent paresthesias, radicular pain). Resolution of late neurologic manifestations of Lyme disease may occur very slowly and, in some patients, may be incomplete.Acrodermatitis chronica atrophicans, a chronic skin lesion characterized by inflammation and subsequent atrophy, is a late manifestation of Lyme disease that has been reported in Europe but only rarely in the United States. Diagnosis of Lyme disease is based principally on clinical findings, and treating patients with early disease solely on the basis of objective signs and a known exposure often is appropriate. Individuals with known endemic exposure to B. burgdorferi and physician-diagnosed erythema migrans can receive treatment for Lyme disease without serologic testing; however, erythema migrans should be clinically differentiated from similar rashes that are not caused by B. burgdorferi infection. In areas of low or no endemic risk, the likelihood of Lyme disease in a patient with a rash resembling erythema migrans is low. Serologic testing can provide valuable supportive diagnostic information in patients with endemic exposure and objective clinical findings that indicate later stage disseminated Lyme disease. Negative test results are useful in ruling out Lyme disease in patients with clinical findings compatible with disseminated or late-stage infection. Since the proportion of false-positive test results increases when the pretest probability of Lyme disease is low, the use of testing to make a diagnosis of Lyme disease in individuals without endemic exposure is not recommended. When serologic testing is indicated to aid in diagnosis, the CDC, Association of State, Territorial, and Public Health Laboratory Directors (ASTPHLD), and other clinicians recommend initial testing with a sensitive screening test, either an enzyme-linked immunosorbent assay (ELISA) or an indirect fluorescent antibody (IFA) test, followed by testing with the more specific Western blot (immunoblot) test to corroborate equivocal or positive results obtained with the initial test. Although anti-infective treatment in early localized disease may blunt or abrogate the antibody response, patients with early disseminated or late-stage disease usually have strong serologic reactivity and demonstrate expanded IgG Western blot banding patterns to diagnostic B. burgdorferi antigens. Antibodies often persist for months or years following successfully treated or untreated infection. Therefore, seroreactivity alone cannot be used as a marker of active disease. Repeated infection with B. burgdorferi has been reported, and neither a positive serologic test result and/or a history of prior Lyme disease ensures that an individual has protective immunity. The CDC and National Institute of Allergy and Infectious Diseases (NIAID) state that clinicians should be familiar with current recommendations for diagnosis and treatment of Lyme disease and should be alert for and know how to minimize potential complications associated with therapy for the disease.
Postexposure Prophylaxis after Tick Bite
In a recent controlled study in adults, a single dose of oral doxycycline was effective in preventing Lyme disease when given within 72 hours after a documented I. scapularis tick bite, and some clinicians suggest that this single-dose antibiotic regimen may be useful for individuals in Lyme-disease endemic areas who are bitten by an I. scapularis tick (particularly a nymphal tick) that is at least partially engorged with blood. However, the accurate and timely identification of tick species or stage of development and infection status of the tick as well as assessment of the degree of tick engorgement are often difficult, and postexposure antibiotic prophylaxis appears unlikely to have a substantial effect on disease incidence since most ticks that are recognized are removed within 48 hours (i.e., usually before transmission of infection). In addition, I. scapularis ticks may transmit other infections such as babesiosis (B. microti) for which doxycycline may not be appropriate therapy. The CDC, IDSA, AAP, and other clinicians currently do not recommend routine anti-infective prophylaxis or serologic testing for individuals after a tick bite. The IDSA states that the best currently available method for preventing B. burgdorferi infection and other Ixodes-transmitted infections is avoidance of tick exposure; if exposure is unavoidable, the risk of infection can be reduced through use of protective clothing and tick repellents, daily checking of the entire body for ticks, and prompt removal of attached ticks before transmission of B. burgdorferi infection. Individuals from whom attached ticks are removed should be closely monitored for 30 days; those who develop a skin lesion at the site of the tick bite, a temperature exceeding 38°C, or other illness within 1 month after removal of an attached tick should receive a prompt assessment for tick-borne diseases, including Lyme disease, human granulocytic ehrlichiosis [HGE], or babesiosis. Patients who have received Lyme disease vaccine (no longer commercially available in the US) have a reduced risk of developing the disease but should be assessed in a similar manner as those who have not been vaccinated against the disease. In a double-blind, placebo-controlled study, individuals 12-82 years of age who resided in a hyperendemic area and who had within the previous 72 hours removed an attached tick that was entomologist-verified as I. scapularis were randomized to receive a single oral dose of doxycycline 200 mg or placebo. At baseline and at 3 and 6 weeks, these individuals were examined for manifestations of B. burgdorferi infection, including erythema migrans; serum antibody levels and blood cultures also were obtained at these time points. Erythema migrans at the site of the tick bite (the primary end point) occurred in 1 of 235 individuals (0.4%) who had received doxycycline versus 8 of 247 individuals (3.2%) who received placebo, representing 87% efficacy for this prophylactic regimen. However, extrapolation of the findings of this study to other clinical settings should take into account that this efficacy rate is based on a relatively small number of individuals who developed Lyme disease and that identification of I. scapularis ticks by patients and/or clinicians may not be presumed to be as accurate as that by the medical entomologists in this study.
Treatment of Early Localized or Disseminated Lyme Disease
Anti-infective therapy usually is effective in all stages of Lyme disease, and appropriate treatment of early disease shortens the duration of symptoms and generally prevents the development of late sequelae. The IDSA, AAP, and other clinicians currently recommend oral doxycycline or oral amoxicillin as first-line therapy for the treatment of early localized or early disseminated Lyme disease associated with erythema migrans, in the absence of neurologic involvement or third-degree atrioventricular (AV) heart block. Cefuroxime axetil is an effective alternative agent that, because of its greater cost, is recommended for patients with early Lyme disease who are allergic to or intolerant of doxycycline and amoxicillin. Although the optimal duration of therapy has not been established, most clinicians treat early Lyme disease for 14-21 days. Oral doxycycline, amoxicillin, or cefuroxime axetil may be preferred to other drugs such as oral tetracycline hydrochloride, oral penicillin G, or oral penicillin V, particularly in patients with early disseminated infection, because of improved microbiologic activity, better GI absorption and tolerance, and/or higher CSF drug concentrations. Macrolide antibiotics (e.g., erythromycin, azithromycin, clarithromycin) also have been used for the treatment of early Lyme disease, although limited evidence suggests that erythromycin or azithromycin may not be as effective as other recommended agents.The IDSA and other clinicians state that macrolide antibiotics are not recommended as first-line therapy for early Lyme disease; these agents should be reserved for patients who are intolerant of amoxicillin, doxycycline, and cefuroxime axetil, and patients treated with macrolides should be monitored closely. Most patients with erythema migrans who received oral doxycycline, amoxicillin, or cefuroxime axetil in multicenter studies had satisfactory outcomes; although subjective symptoms persisted in some patients after treatment, objective evidence of persistent infection or relapse was rare and retreatment usually was unnecessary. In a randomized, controlled study, doxycycline 100 mg twice daily or amoxicillin 500 mg 3 times daily (plus probenecid 500 mg 3 times daily) for 21 days showed similar efficacy in preventing late complications (e.g., meningitis, myocarditis, arthritis) in patients with early Lyme disease (erythema migrans); mild fatigue or arthralgia occurred infrequently following antibiotic therapy but resolved in all cases within the 6-month follow-up period. In a multicenter study, oral therapy with doxycycline 100 mg 3 times daily or cefuroxime 500 mg twice daily for 20 days resulted in cure or improvement in about 90% of patients, and even those considered to have failed therapy did not show objective evidence of continuing infection. Although effective, ceftriaxone is not superior to recommended oral drugs for the treatment of early Lyme disease and is not a recommended first-line agent in the absence of neurologic manifestations or third-degree atrioventricular block. While doxycycline has the advantage of being effective for the treatment of human granulocytic ehrlichiosis (HGE), which may occur concurrently in patients with early Lyme disease, tetracyclines generally are contraindicated in pregnant or nursing women and in children younger than 8 years of age. Transplacental transmission of B. burgdorferi appears to occur rarely, if at all, and epidemiologic studies in pregnant women have not documented an association between exposure to Lyme disease prior to conception or during pregnancy and subsequent fetal death, congenital malformations, or prematurity. The IDSA, AAP, and other clinicians state that pregnant or nursing women need not be treated differently than other patients with Lyme disease, except that they should not receive tetracyclines. For treatment of early Lyme disease in children, the AAP, IDSA, and other clinicians recommend oral doxycycline for children 8 years of age or older and amoxicillin for all other ages. Children who cannot tolerate amoxicillin or doxycycline can be treated with cefuroxime axetil. Limited evidence in patients with early disseminated Lyme disease (e.g., multiple erythema migrans lesions and/or objective evidence of organ involvement [e.g., arthritis, heart block, facial nerve palsy]) who did not have meningitis suggests that oral doxycycline is a cost-effective alternative to ceftriaxone for preventing late manifestations of the disease. In a randomized, comparative study, clinical cure (resolution of objective clinical findings of Lyme disease) was reported in 88 or 85% of patients receiving oral doxycycline (100 mg twice daily for 21 days) or ceftriaxone (2 g IV or IM daily for 14 days), respectively, and both regimens were well tolerated. Although further study is needed to determine the relative safety and efficacy of oral versus IV antibiotic therapy in the treatment of Lyme disease, oral therapy is easier to administer than IV therapy, is associated with fewer serious complications, and is more economical. Patients with facial nerve palsy alone or uncomplicated Lyme arthritis usually respond adequately to prolonged therapy (e.g., 28 days) with oral anti-infectives (e.g., oral doxycycline or amoxicillin). However, more severe or late complications of Lyme disease generally require higher dosages and more prolonged therapy and/or parenteral anti-infectives (e.g., ceftriaxone or alternatively, cefotaxime or IV penicillin G for 14-28 days).
Treatment of Late or Persistent Manifestations of Lyme Disease
Neurologic Manifestations
Patients with early Lyme disease who have acute neurologic involvement manifested by facial nerve palsy alone should receive antibiotic therapy to prevent further sequelae; antibiotic therapy has not been shown to accelerate resolution of palsy. Neurologic examination, including lumbar puncture, should be performed in patients in whom neurologic disease is strongly suspected on clinical grounds. The IDSA states that patients with negative CSF examinations may be treated with the same antibiotic regimens recommended for patients with erythema migrans (i.e., oral doxycycline, amoxicillin, or alternatively, oral cefuroxime axetil); those with clinical and laboratory evidence of CNS involvement should be treated with regimens effective against meningitis. The IDSA states that while evidence from studies in Europe suggests that IV penicillin G and ceftriaxone or cefotaxime have similar efficacy in treating acute neurologic manifestations of Lyme disease, ceftriaxone often is used because its once-daily administration schedule allows outpatient management of therapy. For adults with acute neurologic disease manifested by meningitis or radiculopathy, the IDSA and other clinicians recommend ceftriaxone for 14-28 days; alternatively, IV penicillin G or cefotaxime may be used. Some clinicians suggest that oral or IV doxycycline for 14-28 days may be adequate therapy in adults with acute neurologic manifestations who are intolerant of cephalosporins and penicillin, although experience in the US with such a regimen for Lyme meningitis is limited. Children younger than 8 years of age with acute neurologic manifestations of meningitis or radiculopathy should receive ceftriaxone or cefotaxime for 14-28 days; IV penicillin G may be used as an alternative in such children.
Cardiac Manifestations
Cardiac involvement in Lyme disease usually manifests as atrioventricular (AV) heart block and generally occurs during the first few weeks of infection in conjunction with erythema migrans. The IDSA states that patients with first- or second-degree AV heart block associated with early Lyme disease should be treated with the same antibiotic regimens (i.e., oral doxycycline or amoxicillin) as patients with erythema migrans who do not have carditis. However, some clinicians recommend use of IV regimens (e.g., ceftriaxone, cefotaxime, or penicillin G) in patients with first-degree AV block and a PR-interval exceeding 0.3 seconds. The IDSA and other clinicians recommend that patients with third-degree AV heart block be hospitalized for cardiac monitoring because of the potential for life-threatening complications. While evidence supporting the superiority of IV versus oral therapy currently is unavailable, most clinicians recommend that patients with severe cardiac involvement receive ceftriaxone, penicillin G, or cefotaxime IV for 14-28 days. Patients with third-degree AV block may require a temporary pacemaker.
Lyme Arthritis
Patients with uncomplicated Lyme arthritis generally can be treated with a prolonged course (e.g., 28 days) of oral anti-infectives (i.e., doxycycline or amoxicillin). While oral therapy is easier to administer than IV antibiotics, some patients with Lyme arthritis treated with oral regimens have subsequently developed overt neuroborreliosis, which may require IV therapy for successful treatment. When Lyme arthritis is accompanied by neurologic disease documented by CSF analysis, the IDSA and other clinicians recommend that adults receive ceftriaxone for 14-28 days; alternative therapy is cefotaxime or IV penicillin G. Ceftriaxone or cefotaxime is recommended in children with coexisting Lyme arthritis and neurologic manifestations; alternatively, IV penicillin G may be given. Long-acting penicillin G benzathine preparations are not recommended for treatment of these patients because of the low serum penicillin G concentrations attained after administration of such preparations.
Treatment of Late or Persistent Manifestations of Lyme Disease.
Late manifestations of Lyme disease may include oligoarticular arthritis, encephalopathy (principally memory deficit, irritability, and somnolence), and neuropathy (principally distal paresthesias or radicular pain). Comparative studies evaluating different antibiotic regimens in patients with late Lyme disease generally are lacking. Response to therapy for late manifestations of Lyme disease may be slow and improvement may take weeks or months, although most appropriately treated patients eventually recover.
Persistent Arthritis
In patients who have persistent or recurrent joint swelling after receiving recommended antibiotic regimens, the IDSA and other clinicians recommend another 28-day course of a recommended oral antibiotic or 14-28 days of ceftriaxone, cefotaxime, or IV penicillin G. However, the IDSA states that clinicians should consider allowing several months for joint inflammation to resolve after initial treatment before an additional course of antibiotic therapy is given. Many clinicians also suggest that the initial diagnosis of Lyme disease be reevaluated in patients who appear not to respond to recommended anti-infective therapy (e.g., 21-28 days of oral and/or IV antibiotics). Patients with persistent arthritis who have received 2 courses of recommended oral antibiotic therapy or a single course of IV antibiotics should receive symptomatic treatment with a nonsteroidal anti-inflammatory agent (NSAIA); treatment with intra-articular corticosteroids also may be considered. Arthroscopic synovectomy may be indicated and may decrease time to recovery in patients with persistent synovitis who report substantial pain or limited function.
Late Neuroborreliosis Affecting the CNS or Peripheral Nervous System.
Patients with late neurologic disease affecting the CNS or peripheral nervous system (e.g., encephalopathy, neuropathy) should receive 14-28 days of ceftriaxone; alternative therapy is cefotaxime or IV penicillin G. Clinical response to these regimens is typically gradual and may be incomplete. However, additional courses of antibiotic therapy are not recommended unless reliable objective measures substantiate relapse of neurologic disease. For children with late CNS or peripheral nervous system manifestations, ceftriaxone for 14-28 days is recommended; alternatively, cefotaxime or IV penicillin G may be given.
Chronic Lyme Disease or Post-Lyme Disease Syndrome
After receiving recommended antibiotic therapy for Lyme disease, some patients manifest a post-infectious syndrome, which some clinicians have referred to as chronic Lyme disease or post-Lyme disease syndrome. Some evidence suggests that this syndrome occurs more frequently in patients who have symptoms of early dissemination of the infection, particularly if there is a delay in treatment. Patients with this syndrome are a diverse group and report various persistent subjective complaints including arthralgia, fatigue, and/or myalgia; some clinicians categorize such symptoms as chronic Lyme disease or a post-Lyme disease syndrome similar to chronic fatigue syndrome or fibromyalgia. Patients with residual B. burgdorferi infection (or possibly reinfection) have been reported rarely in Europe; however, residual infection has not been reliably documented to date in a large series of patients in Europe or North America who received appropriate treatment for Lyme disease. The IDSA states that currently available evidence is insufficient to consider chronic Lyme disease a separate diagnostic entity. Although case reports and uncontrolled studies have reported benefit from prolonged antibiotic therapy in patients with chronic Lyme disease, symptom recurrence after discontinuance of therapy is common; in addition, the prevalence of fatigue and/or arthralgias in the general population reportedly is greater than 10%. In a small percentage of patients appropriately treated for Lyme disease, persistent symptoms may be explained by coinfection with B. microti or Ehrlichia spp. (HGE). Although studies of treatment of patients with Lyme disease who remain unwell after standard courses of antibiotic therapy currently are in progress, the IDSA states that no controlled clinical studies currently support the efficacy of repeated or prolonged courses of oral and/or IV antibiotics in such patients. In addition, serious complications (e.g., biliary disease leading to cholecystectomy), including at least one death, have been reported in patients receiving such empiric therapy. The American College of Rheumatology and IDSA state that the risks and costs of treating suspected Lyme disease empirically with IV antibiotics (e.g., ceftriaxone) exceed the benefits in patients with a positive antibody titer for B. burgdorferi and only nonspecific complaints of myalgia or fatigue; such patients are best managed symptomatically rather than with prolonged courses of antibiotics. Two double-blind, placebo-controlled studies evaluating prolonged antibiotic therapy (IV ceftriaxone followed by oral doxycycline for a total of 90 days) in patients with persistent symptoms of Lyme disease after appropriate initial therapy were discontinued after interim analysis revealed no difference in clinical benefit between prolonged antibiotic therapy and placebo. In these 2 studies, patients were randomized to receive IV ceftriaxone 2 g daily for 30 days followed by oral doxycycline 100 mg twice daily for 60 days, or matching IV and oral placebos. Patients were enrolled in the 2 studies based on their seropositivity or seronegativity for antibodies to B. burgdorferi antigen on Western blot assay. All patients included in the studies had one or more of the following diagnoses: a history of single or multiple erythema migrans skin lesions, early neurologic or cardiac symptoms attributed to Lyme disease, radiculoneuropathy, or Lyme arthritis; seronegative patients were required to have a documented history of erythema migrans by an experienced clinician. These patients were experiencing profound fatigue, widespread musculoskeletal pain, cognitive impairment, radicular pain, paresthesias, and/or dysesthesias that had begun within 6 months of the initial documented diagnosis of US-acquired acute Lyme disease and had persisted for at least 6 months but less than 12 years. Most patients in these studies previously had received oral antibiotic therapy for a median of 50 days; 33% had received IV antibiotic therapy for an average of 30 days. Baseline evaluation included a complete review of patient medical history, physical examination, neuropsychologic testing, CSF analysis, and phlebotomy; cultures and molecular analysis by polymerase chain reaction (PCR) of blood and CSF at baseline or during the studies did not detect any evidence of persistent infection by B. burgdorferi in these patients. A planned interim analysis of data from the combined studies at 180 days did not reveal a significant difference in the efficacy of prolonged antibiotic therapy compared with that of placebo based on summary scores for the mental and physical components of a health-related quality of life survey instrument (Medical Outcomes Study 36-item Short-Form General Health Survey); separate analysis of data from the seropositive and the seronegative studies also failed to show any benefit of the prolonged antibiotic regimen compared to placebo. In an observational study of patients diagnosed as having chronic Lyme disease (i.e., manifesting symptoms for greater than 3 months from at least 2 out of the following 3 groups of symptoms: fatigue, neurologic complaints [e.g., paresthesias, cognitive dysfunction, radicular pain], musculoskeletal complaints [e.g., arthralgias, myalgias, weakness]), 70% of patients reported improvement and 20% reported resolution of symptoms following long-term therapy (generally 3-6 months) with tetracycline hydrochloride (500 mg 3 times daily). In this study, patients with a longer duration of Lyme disease symptoms prior to receiving tetracycline therapy had a slower onset of improvement following initiation of therapy. Additional study is needed to establish the value of prolonged (e.g., 3-6 months) anti-infective therapy in patients with persistent manifestations of Lyme disease.
Other Spirochetal Infections
Tetracyclines are considered the drugs of choice for the treatment of tick-borne (endemic) or louse-borne (epidemic) relapsing fever caused by Borrelia. The drugs also appear to be effective in the treatment of leptospirosis, yaws, pinta, and bejel and are used as alternatives to penicillin G for the treatment of these diseases. The CDC recommends that leptospirosis be treated with penicillin, ampicillin, or doxycycline; IV penicillin G or ampicillin is indicated in patients with severe disease.
GI Infections
Helicobacter pylori Infection Tetracycline hydrochloride is used in combination with metronidazole, bismuth subsalicylate, and an H2-receptor antagonist for the treatment of Helicobacter pylori (formerly Campylobacter pylori or C. pyloridis) infection in patients with an active duodenal ulcer. Tetracycline hydrochloride also has been used successfully in other multiple-drug regimens (with or without bismuth salts, metronidazole, and/or an H2-receptor antagonist) for the treatment of H. pylori infection in patients with peptic ulcer disease. Current epidemiologic and clinical evidence supports a strong association between gastric infection with H. pylori and the pathogenesis of duodenal and gastric ulcers; long-term H. pylori infection also has been implicated as a risk factor for gastric cancer. For additional information on the association of this infection with these and other GI conditions. Conventional antiulcer therapy with H2-receptor antagonists, proton-pump inhibitors, sucralfate, and/or antacids heals ulcers but generally is ineffective in eradicating H. pylori, and such therapy is associated with a high rate of ulcer recurrence (e.g., 60-100% per year). Several useful therapeutic regimens for H. pylori-associated peptic ulcer disease have been identified, and the American College of Gastroenterology (ACG), the National Institutes of Health (NIH), and most clinicians currently recommend that all patients with initial or recurrent duodenal or gastric ulcer and documented H. pylori infection receive anti-infective therapy for treatment of the infection. The optimum regimen for treatment of H. pylori infection has not been established; however, combined therapy with 3 drugs that have activity against H. pylori (generally a bismuth salt, metronidazole, and tetracycline or amoxicillin) has been effective in eradicating the infection, resolving associated gastritis, healing peptic ulcer, and preventing ulcer recurrence in many patients with H. pylori-associated peptic ulcer disease. Although such 3-drug regimens typically have been administered for 10-14 days, current evidence principally from studies in Europe suggests that 1 week of such therapy provides H. pylori eradication rates comparable to those of longer treatment periods. Other regimens that combine one or more anti-infective agents (e.g., clarithromycin, amoxicillin) with a bismuth salt and/or an antisecretory agent (e.g., omeprazole, H2-receptor antagonist) also have been used successfully for H. pylori eradication, and the choice of a particular regimen should be based on the rapidly evolving data on optimal therapy, including consideration of the patient’s prior exposure to anti-infective agents, the local prevalence of resistance, patient compliance, and costs of therapy. Current data suggest that eradication of H. pylori infection using regimens consisting of 1 or 2 anti-infective agents with a bismuth salt and/or an H2-receptor antagonist or proton-pump inhibitor (e.g., omeprazole, lansoprazole) is cost effective compared with intermittent or continuous maintenance therapy with an H2-receptor antagonist (considering the costs associated with ulcer recurrence, including endoscopic or other diagnostic procedures, physician visits, and/or hospitalization). Although high eradication rates have been achieved with standard 3-drug, bismuth-based regimens (e.g., bismuth-metronidazole-tetracycline or bismuth-metronidazole-amoxicillin), such regimens typically involve administration of many tablets/capsules and have been associated with a relatively high (although variable) incidence of adverse effects. In addition, the efficacy of these regimens generally is unacceptable in patients with H. pylori strains resistant to the imidazole anti-infective (e.g., metronidazole) component. Current evidence suggests that inclusion of a proton-pump inhibitor (e.g., omeprazole, lansoprazole) in anti-H. pylori regimens containing 2 anti-infectives enhances effectiveness, and limited data suggest that such regimens retain good efficacy despite imidazole (e.g., metronidazole) resistance. Therefore, the ACG and many clinicians currently recommend 1 week of therapy with a proton-pump inhibitor and 2 anti-infective agents (usually clarithromycin and amoxicillin or metronidazole), or a 3-drug, bismuth-based regimen (e.g., bismuth-metronidazole-tetracycline) concomitantly with a proton-pump inhibitor, for treatment of H. pylori infection. Although few comparative studies have been performed, such regimens appear to provide high (e.g., 85-90%) H. pylori eradication rates, are well tolerated, and may be associated with better patient compliance than more prolonged therapy. The ACG states that in a cost-sensitive environment, an alternative regimen consisting of a bismuth salt, metronidazole, and tetracycline for 14 days is a reasonable choice in patients who are compliant and in whom there is a low expectation of metronidazole resistance (no prior exposure to the drug and a low regional prevalence of resistance). Rapid development of resistance by H. pylori to certain drugs (e.g., metronidazole, clarithromycin and other macrolides, quinolones) has occurred when these drugs were used as monotherapy or as the only anti-infective agent in anti-H. pylori regimens. Resistance commonly emerges during therapy with clarithromycin or metronidazole when eradication of H. pylori is not achieved; therefore, prior exposure to these anti-infectives predicts resistance in individual patients and should be considered when selecting anti-H. pylori treatment regimens. Regimens containing metronidazole or clarithromycin should not be used to treat H. pylori infection in patients with known or suspected metronidazole- or clarithromycin-resistant isolates because of reduced efficacy in such patients. For additional discussion of H. pylori infection, including details about the efficacy of various regimens and rationale for drug selection.
Vibrio Infections
Cholera Tetracyclines (doxycycline, tetracycline hydrochloride) usually are considered the drugs of choice when anti-infective therapy is indicated as an adjunct to fluid and electrolyte replacement in patients with cholera. Although some clinicians recommend use of co-trimoxazole for the treatment of cholera in children younger than 8 years of age, the AAP states that in cases of severe cholera, the benefits of tetracyclines may outweigh the risks in this age group. Tetracycline therapy reduces the duration of excretion of Vibrio cholerae and, since it decreases the volume and duration of diarrhea, may decrease requirements for fluid replacement. Adjunctive anti-infective therapy should be considered in patients with moderately to severe disease. When the infection is caused by strains of V. cholerae resistant to tetracyclines, alternative agents include co-trimoxazole, fluoroquinolones, or furazolidone.
Vibrio parahaemolyticus Infections
Tetracyclines (doxycycline, tetracycline hydrochloride) are one of several alternatives recommended for the treatment of severe cases of Vibrio parahaemolyticus infection when anti-infective therapy is indicated in addition to supportive care. V. parahaemolyticus infection is a relatively rare foodborne illness than can occur as the result of ingestion of contaminated, undercooked or raw fish or shellfish; the incubation period usually is 2-48 hours. The signs and symptoms of V. parahaemolyticus infection are watery diarrhea, abdominal cramps, and nausea and vomiting lasting 2-5 days. Although supportive care usually is sufficient, some clinicians recommend use of tetracycline, doxycycline, gentamicin, or cefotaxime in severe cases.
Vibrio vulnificus Infections
Some clinicians suggest that tetracyclines (doxycycline, tetracycline hydrochloride) are drugs of choice for the treatment of infections caused by Vibrio vulnificus. V. vulnificus, a gram-negative aerobic bacteria that can cause potentially fatal septicemia, wound infections, or gastroenteritis, generally is transmitted through ingestion of contaminated raw or undercooked seafood (especially raw oysters) or through contamination of a wound with seawater or seafood drippings. V. vulnificus is naturally present in marine environments, thrives in warm ocean water, and frequently is isolated from oysters and other shellfish harvested from the Gulf of Mexico and from US coastal waters along the Pacific and Atlantic ocean. Individuals with preexisting liver disease are at high risk for developing fatal septicemia following ingestion of seafood contaminated with V. vulnificus and debilitated or immunocompromised individuals (e.g., those with chronic renal impairment, cancer, diabetes mellitus, steroid-dependent asthma, chronic GI disease) or individuals with iron overload states (e.g., thalassemia and hemochromatosis) also are at increased risk for fatal infections. In immunocompromised individuals, fever, nausea, myalgia, and abdominal cramps may occur as soon as 24-48 hours after ingestion of seafood contaminated with V. vulnificus and sepsis and cutaneous bullae may be present within 36 hours of the onset of symptoms. Because the case fatality rate for V. vulnificus septicemia exceeds 50% in immunocompromised individuals or those with preexisting liver disease, these individuals should be informed about the health hazards of ingesting raw or undercooked seafood (especially oysters), the need to avoid contact with seawater during the warm months, and the importance of using protective clothing (e.g., gloves) when handling shellfish. V. vulnificus should be considered in the differential diagnosis of fever of unknown etiology, and individuals who present with fever (especially when bullae, cellulitis, or wound infection is present) and who have preexisting liver disease or are immunocompromised should be questioned regarding a history of raw oyster ingestion or seawater contact. While optimum anti-infective therapy for the treatment of V. vulnificus infections has not been identified, use of a tetracycline or third generation cephalosporin (e.g., cefotaxime, ceftazidime) is recommended. Because the high fatality rate associated with V. vulnificus infections, anti-infective therapy should be initiated promptly if indicated.
Yersinia Infections
Tetracyclines (usually doxycycline) are suggested as possible choices for the treatment of GI infections caused by Yersinia enterocolitica or Y. pseudotuberculosis. (For information on treatment of Y. pestis infections, see Plague under Uses: Gram-negative Bacterial Infections.) Y. enterocolitica and Y. pseudotuberculosis GI infections usually are self-limited and anti-infective therapy unnecessary; however, the AAP, IDSA, and others recommend use of anti-infectives in immunocompromised individuals or for the treatment of severe infections or when septicemia or other invasive disease occurs. GI infections caused by Y. enterocolitica or Y. pseudotuberculosis can occur as the result of ingesting undercooked pork, unpasteurized milk, or contaminated water; infection has occurred in infants whose caregivers handled contaminated chitterlings (raw pork intestines) or tofu. Use of co-trimoxazole, an aminoglycoside (amikacin, gentamicin, tobramycin), a fluoroquinolone (e.g., ciprofloxacin), doxycycline, cefotaxime, or ceftizoxime has been recommended when treatment is considered necessary; combination therapy may be necessary. Some clinicians suggest that the role of oral anti-infectives in the management of enterocolitis, pseudoappendicitis syndrome, or mesenteric adenitis caused by Yersinia needs further evaluation.
Travelers’ Diarrhea
Although doxycycline has been used in the past for the treatment of travelers’ diarrhea, other anti-infective agents (i.e., ciprofloxacin, levofloxacin, norfloxacin, ofloxacin, levofloxacin, azithromycin) are preferred if anti-infective therapy is indicated because diarrhea is severe or associated with fever or bloody stools.
Other GI Infections
Tetracycline hydrochloride is considered the treatment of choice for balantidiasis caused by Balantidium coli; metronidazole and iodoquinol are considered alternative agents. Although the manufacturers state that tetracyclines may be useful as an adjunct to amebicides in the treatment of acute intestinal amebiasis, tetracyclines are not generally recommended for the treatment of amebiasis caused by Entamoeba.Tetracyclines are recommended as alternatives to iodoquinol for the treatment of infections caused by Dientamoeba fragilis. Tetracycline hydrochloride is used in conjunction with folic acid in the treatment of tropical sprue (postinfectious tropical malabsorption). Although treatment with folic acid alone can improve symptoms of tropical sprue, it does not cure the diarrhea; combination therapy appears to be most effective in resolving symptoms (including diarrhea) and promoting weight gain. For the treatment of Whipple’s disease caused by Tropheryma whippelii, some clinicians suggest that co-trimoxazole is the drug of choice and penicillin G and tetracyclines are alternatives.
Malaria
Prevention of Malaria
Doxycycline is used for prevention of malaria in individuals traveling to areas where chloroquine-resistant Plasmodium falciparum have been reported. For prevention of malaria in individuals traveling to malarious areas where chloroquine-resistant P. falciparum has been reported, the CDC and many clinicians recommend use of the fixed-combination of atovaquone and proguanil hydrochloride (Malarone®), doxycycline, or mefloquine. Doxycycline (unless contraindicated) can be used for prophylaxis in individuals traveling to areas of risk where mefloquine resistance has been confirmed (e.g., Thai-Cambodia and Thai-Burma border areas, western Cambodia, eastern Burma). Because doxycycline is active only against the asexual erythrocytic forms of P. falciparum, the drug provides substantial, but not complete, suppressive action for the plasmodia. Specific references, including those most recently published by the CDC and World Health Organization (WHO), should be consulted to determine in which countries a risk of acquiring malaria exists, to determine whether prophylaxis against malaria is indicated in these countries, and to aid in selecting the appropriate antimalarial agent(s). The choice of an antimalarial agent(s) for suppression or chemoprophylaxis depends on the specific malarious area as well as the duration of exposure. No drug regimen is completely effective in preventing malaria, and travelers should be informed that, regardless of the prophylactic regimen used, it is still possible to contract malaria. Travelers should be advised of the importance of taking measures to reduce contact with mosquitoes (e.g., using appropriate insect repellents, wearing clothes that cover most of the body, remaining in air-conditioned or well-screened areas, using mosquito nets [e.g., bed nets] between dusk and dawn, using aerosolized insecticides in rooms where mosquitoes are found). Travelers should purchase insect repellent before travel. The most effective insect repellents contain diethyltoluamide (DEET). The concentration of DEET varies considerably among commercially available repellants and can be as high as 95%. The CDC states that repellants with DEET concentrations of 30-35% are quite effective, and the effect should last about 4 hours. Some clinicians suggest that products with 10-35% DEET are effective for adults and higher concentrations do not improve efficacy. Long-acting (controlled release) DEET products containing 20-33% DEET also are commercially available and can provide protection for 6-12 hours. Some individuals (e.g., children) exposed to DEET have had severe adverse reactions including toxic encephalopathy. The possibility of adverse reactions to DEET can be minimized by applying the repellent sparingly only to exposed skin or clothing; avoiding use of high-concentration products on the skin, particularly in children; avoiding inhaling or ingesting repellents; avoiding contact with the eyes, mouth, wounds, or irritated skin; and washing repellent-treated skin after coming indoors. The AAP has recommended that repellents for children contain no more than 10% DEET. Travelers should be instructed to wash the skin and seek medical attention if a suspected reaction to insect repellent occurs. Travelers also should purchase a pyrethroid-containing flying-insect spray for use in living and sleeping areas during evening and nighttime hours. Spraying clothing with permethrin (Duranon®, Permanone®) and using permethrin- or deltamethrin- (not commercially available in the US) impregnated mosquito nets also may help provide protection against mosquitoes. Permethrin sprayed on fabric remains effective for several weeks (through at least 5 or 6 launderings) and can be used in conjunction with DEET for increased protection. DEET may decrease the effectiveness of sunscreens, and a higher sunscreen protection factor (SPF) should be used if a sunscreen and a product containing DEET are used concomitantly. Travelers to countries with malaria should be instructed to seek prompt medical attention if they develop symptoms of malaria, including fever with chills and headache, while traveling or for up to 3 years after return, especially during the first 2 months. Malaria symptoms can develop as early as 6 days after initial exposure or can appear months after departure from a malarious area, even after chemoprophylaxis is discontinued. Travelers should understand that malaria can be effectively treated early in the course of the disease, but that delays before initiation of therapy can have serious or even fatal consequences.
Treatment of Malaria
Doxycycline or tetracycline hydrochloride is used in conjunction with quinine sulfate for the treatment of chloroquine-resistant P. falciparum malaria. Doxycycline also is used in conjunction with quinine sulfate for the treatment of chloroquine-resistant P. vivax malaria.
Mycobacterial Infections
Leprosy
Minocycline is used as an alternative agent in multiple-drug regimens used for the treatment of multibacillary leprosy and also is used in a single-dose rifampin-based multiple-drug regimen for the treatment of single-lesion paucibacillary leprosy. For the treatment of multibacillary leprosy (i.e., more than 5 lesions or skin smear positive for acid-fast bacteria), the World Health Organization (WHO) currently recommends a multiple-drug regimen that includes rifampin, clofazimine, and dapsone. Minocycline is recommended as an alternative for use in antileprosy regimens in patients with multibacillary leprosy who will not accept or cannot tolerate clofazimine, or when rifampin cannot be used because of adverse effects, intercurrent disease (e.g., chronic hepatitis), or infection with rifampin-resistant Mycobacterium leprae. For the treatment of paucibacillary leprosy (i.e., 2-5 skin lesions), the WHO usually recommends a 6-month multiple-drug regimen that includes rifampin and dapsone. However, patients with single-lesion paucibacillary leprosy (i.e., a single skin lesion with definite loss of sensation but without nerve trunk involvement) have been effectively treated with a single-dose rifampin-based multiple-drug regimen (ROM) that includes a single dose of rifampin, a single dose of ofloxacin, and single dose of minocycline. The single-dose ROM regimen may be an acceptable and cost-effective alternative regimen in antileprosy programs that have detected a large number of patients (e.g., more than 1000 annually) with single-lesion paucibacillary leprosy; however, the WHO states that the single-dose ROM regimen should not be used in antileprosy programs that have detected few single-lesion paucibacillary leprosy patients since it involves additional logistic and informational problems for these programs.
Other Mycobacterial Infections
Minocycline or doxycycline are considered drugs of choice for the treatment of cutaneous infections caused by Mycobacterium marinum.
Ehrlichia Infections
Doxycycline is considered the drug of choice for the treatment of ehrlichiosis caused by Ehrlichia chaffeensis, E. canis, E. ewingii, or E. phagocytophila. While optimum anti-infective regimens for the treatment of ehrlichiosis have not been established, the CDC, AAP, and others suggest that empiric therapy with doxycycline be initiated promptly if ehrlichiosis is suspected since a delay in treatment while awaiting laboratory confirmation of the diagnosis may increase the risk for adverse outcomes. Some clinicians suggest chloramphenicol as an alternative for the treatment of infections caused by E. chaffeensis and rifampin as an alternative for the treatment of infections caused by E. phagocytophila.
Periodontitis
Oral doxycycline (administered as 20-mg tablets) is used as an adjunct to scaling and root planing to promote attachment level gain and to reduce pocket depth in adults with periodontitis. Doxycycline (administered subgingivally as a controlled-release preparation) or minocycline (administered subgingivally as sustained-release microspheres) is used in conjunction with scaling and root planing for the reduction of pocket depth in adults with periodontitis.
Rheumatoid Arthritis
Minocycline is used in the treatment of rheumatoid arthritis. Results of one study indicate that minocycline is at least as effective as hydroxychloroquine in the management of rheumatoid arthritis in adults. Minocycline is one of several disease-modifying antirheumatic drugs (DMARDs) that can be used when DMARD therapy is appropriate.
Syndrome of Inappropriate Antidiuretic Hormone Secretion
Demeclocycline (but not other currently available tetracyclines) has been effective when used in the treatment of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Because it takes several days for diuresis to occur after initiation of demeclocycline therapy, the drug is of limited value in patients with acute water intoxication caused by excess ADH secretion. However, demeclocycline is effective in inhibiting the action of ADH in patients with the chronic form of the disease, and most clinicians advocate the use of the drug instead of lithium. Further studies are needed to compare the safety and efficacy of demeclocycline and other forms of therapy for SIADH. Demeclocycline has also been used to treat hyponatremia and water retention in patients with congestive heart failure or cirrhosis. However, use of demeclocycline in these conditions has been associated with a high incidence of renal failure, and the drug probably should not be used in these patients.
Pericardial and Pleural Effusions
Tetracycline hydrochloride (no longer commercially available as a parenteral formulation in the US) has been used by intracavitary injection as a sclerosing agent to control pleural effusions caused by metastatic tumors. Doxycycline also has been administered in a limited number of patients by intracavitary injection or via lavage drainage for the management of malignant pleural effusions, and minocycline has been administered by intracavitary injection for malignant pleural effusions and concurrent postoperative air leaks in a few patients. Patients with malignant pleural effusions frequently have symptoms of dyspnea, cough, and chest pain and heaviness. Although thoracentesis may provide temporary relief of such symptoms, the effusion often reaccumulates rapidly, and surgical insertion of a thoracostomy tube with subsequent intrapleural instillation of a sclerosing agent generally is considered the treatment of choice for such effusions in patients with neoplasms unresponsive to systemic antineoplastic or radiation therapy. When instilled into the pleural space, tetracyclines and other effective sclerosing agents cause inflammation that results in fibrosis and adherence of serosal surfaces (pleurodesis), thereby obliterating the pleural space and reducing the chance of fluid reaccumulation; however, most current pleurodesis procedures for malignant pleural effusion appear to be associated with a substantial risk of recurrence. Some clinicians recommend removal of the sclerosing fluid after 0.5-4 hours, although it has been suggested that dispersal of the sclerosing agent and pleurodesis may occur within minutes after instillation of tetracycline. Further studies are needed to elucidate fully the optimum length of time that the drug should remain in the pleural cavity. Pleurodesis appears to be most effective when drainage from the thoracostomy tube does not exceed 100 mL/hour and is unlikely to be successful if the patient’s underlying disease prevents complete expansion of the lung following drainage of the effusion. The most common adverse effects reported with intracavitary administration of tetracyclines into the pleural space are chest pain and fever. Opiate analgesics may be administered prior to the procedure to relieve pain associated with pleurodesis; lidocaine also has been instilled into the chest tube prior to pleurodesis to help alleviate discomfort. Although adverse effects attributable to systemic absorption of doxycycline or minocycline appear to be minimal with currently used intrapleural doses of these drugs, therapeutic serum concentrations of tetracycline and lidocaine have been achieved in some patients following intrapleural administration. Doxycycline or minocycline has been suggested as an alternative to tetracycline hydrochloride for the management of malignant pleural effusions when intrapleural therapy is indicated. Data in a limited number of patients suggest that intrapleural doxycycline prevents pleural fluid reaccumulation in most patients with metastatic pleural effusions, at least in the short term (e.g., 1 month) and appears to be associated with relatively few adverse effects (e.g., chest pain, fever). Another agent, bleomycin, has been reported in at least one controlled study to be more effective and possibly better tolerated but more expensive than tetracycline hydrochloride for the management of such effusions. The efficacy and safety of intrapleural doxycycline or minocycline compared with intrapleural administration of bleomycin or other potentially more toxic and/or complicated therapies (e.g., thoracostomy, talc insufflation, nitrogen mustard) for the management of malignant pleural effusions remains to be determined. Tetracycline hydrochloride and doxycycline also have been administered by intrapericardial injection in a limited number of patients with malignant pericardial effusions and associated cardiac tamponade.
Diagnostic Uses
Because tetracyclines have an affinity for and localize in tumors and necrotic or ischemic tissue, the drugs have been used to detect malignant cells in gastric washings, pleural fluid, and ascitic fluid. After several days of oral tetracycline therapy, appropriate specimens are obtained and examined under ultraviolet light; malignant cells in the samples exhibit a bright yellow-gold fluorescence. In addition, radiolabeled tetracycline hydrochloride has been used to detect tumors, especially in the chest, and to detect and measure the extent of ischemia following myocardial infarcts.
Prophylaxis in Victims of Sexual Assault
Oral doxycycline is used in conjunction with oral metronidazole and IM ceftriaxone for empiric anti-infective prophylaxis in adult or adolescent victims of sexual assault; postexposure hepatitis B vaccination also is recommended for susceptible victims. Many experts recommend routine empiric prophylactic therapy after a sexual assault, and use of such prophylaxis probably benefits most patients since follow-up of assault victims can be difficult and such prophylaxis allays the patient’s concerns about possible infection. Trichomoniasis, genital chlamydial infection, gonorrhea, and bacterial vaginosis are the sexually transmitted diseases most commonly diagnosed in women following sexual assault; however, the prevalence of these infections is substantial among sexually active women and their presence after assault does not necessarily indicate that the infections were acquired during the assault. Chlamydial and gonococcal infections among females are of special concern because of the possibility of ascending infection. When empiric anti-infective prophylaxis is indicated in adult or adolescent sexual assault victims, the CDC recommends administration of a single 125-mg IM dose of ceftriaxone given in conjunction with a single 2-g oral dose of metronidazole and a single 1-g oral dose of azithromycin or a 7-day regimen of oral doxycycline (100 mg twice daily). This 3-drug regimen provides coverage against gonorrhea, chlamydia, trichomoniasis, and bacterial vaginosis, but efficacy in preventing these infections after sexual assault has not been specifically evaluated. Because of possible adverse GI effects with the 3-drug regimen, the CDC suggests that the patient be counseled regarding the possible benefits, as well as the possibility of toxicity of such prophylaxis. Alternative regimens may be required for some patients because of the likelihood of transmission of other sexually transmitted diseases from the assailant. CDC states that a recommendation concerning the appropriateness of antiretroviral prophylaxis against HIV cannot be made based on currently available information, and the decision to offer such prophylaxis should be individualized taking into account the probability of HIV transmission from a single act of intercourse and the nature of the assault (e.g., extent of physical trauma and exposure to ejaculate). There are few data available to establish the risk of a child acquiring a sexually transmitted disease as a result of sexual assault or abuse. The risk is believed to be low in most circumstances, although documentation to support this position is inadequate. The CDC currently states that presumptive treatment for children who have been sexually assaulted or abused is not widely recommended because girls appear to be at lower risk for ascending infection than adolescent or adult women and regular follow-up usually can be ensured. Even if the risk is perceived by the health-care provider to be low, some children or their parents or guardians may have concerns about the possibility of the child contracting a sexually transmitted disease as a result of the assault and these concerns may be an appropriate indication for presumptive treatment in some settings.




