Buy Diflucan Online With Low Price
Support Drug Guide: purchase the best generic medicine from our sponsor, online pharmacy store, where you can place an order and buy generic Diflucan online over the counter at lowest prices, worldwide delivery. Prices for Diflucan (Fluconazole) according to the dosage forms and number of pills. The more pills in a package, the lower the price for 1 pill!
 |
 |
 |
 |
 |
 |
 |
 |
 |
Buy Fluconazole Online
Fluconazole is authorised in the world under the following brand names: Biocanol, Biozolene, Diflucan, Elazor, Flucazol, Flucostat, Flukezol, Flunizol, Flusol, Pritenzol, Triflucan.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Pharmacopoeias
European Pharmacopoeia, 6th ed. (Fluconazole)
A white or almost white, hygroscopic, crystalline powder. It exhibits polymorphism. Slightly soluble in water, freely soluble in methyl alcohol, soluble in acetone. Store in airtight containers.
The United States Pharmacopeia 31, 2008, and Supplements 1 and 2 (Fluconazole)
A white or almost white, crystalline powder. Slightly soluble in water, soluble in alcohol and in acetone, sparingly soluble in chloroform and in isopropyl alcohol, freely soluble in methyl alcohol, and very slightly soluble in toluene. Store in airtight containers at a temperature below 30°.
Most drug interactions with azole antifungals occur through one of two basic mechanisms: impairment of the azole compound’s absorption, leading to reduced blood concentrations, or interference with hepatic microsomal enzymes, altering the metabolism and blood concentrations of the azole, the interacting drug, or both.
Unlike itraconazole and ketoconazole, absorption of fluconazole is not reduced if given together with drugs that reduce gastric acid secretion. Concomitant administration of fluconazole and rifampicin has resulted in a modest reduction in blood levels of the antifungal agent. The effect is less marked than with itraconazole or ketoconazole. This is due to the induction of P-450 cytochrome oxidases by rifampicin, which enhances the azole drug’s hepatic metabolism.
Like rifampicin, phenytoin undergoes cytochrome P-450-mediated hepatic metabolism, and its concomitant administration with fluconazole may reduce its clearance. If these drugs are given together, serum phenytoin concentrations should be monitored, and the dose should be adjusted to maintain therapeutic levels.
Fluconazole has been shown to prolong the serum half-life, thus augmenting the hypoglycaemic effects of chlorpropamide, glibenclamide, glipizide, and tolbutamide. It can also increase the serum concentration of warfarin, augmenting its anticoagulant effects. Therefore, it is recommended that prothrombin time be carefully monitored in patients receiving concomitant treatment with fluconazole and anticoagulants.
Fluconazole may prolong the half-life of cyclosporin in transplant recipients, leading to increased blood levels of that drug. Serum concentrations of cyclosporin should be monitored if these drugs are given together.
Uses
Before buy Fluconazole online, read information about the drug
Fluconazole is used in the treatment of oropharyngeal, esophageal, or vulvovaginal candidiasis and in the treatment of other serious systemic candidal infections (e.g., urinary tract infections, peritonitis, candidemia, disseminated candidiasis, meningitis, pneumonia). The drug is also used for treating meningitis caused by Cryptococcus neoformans and for treating blastomycosis, coccidioidomycosis, and histoplasmosis.
Fluconazole has been used for less severe infections, such as superficial fungal infections, dermatophytoses, and onychomycosis. In addition, the drug is used for the prevention of serious fungal infections (e.g., coccidioidomycosis, cryptococcosis, mucocutaneous candidiasis) in patients with human immunodeficiency virus (HIV) infection and the prevention of fungal infections in other immunocompromised individuals (e.g., cancer patients, bone marrow transplant patients).
Before initiating fluconazole therapy, appropriate specimens for fungal culture and other relevant laboratory studies (e.g., serology, histopathology) should be obtained to isolate and identify the causative organism(s). Fluconazole therapy may be started pending the results of these in vitro tests; however, once results are available, therapy should be adjusted accordingly. If fluconazole in vitro susceptibility tests are performed, results should be interpreted cautiously since currently available tests may not accurately reflect fluconazole’s in vivo activity.
Candidal Infections
Oropharyngeal and Esophageal Candidiasis
Oral or IV fluconazole is used in the treatment of oropharyngeal candidiasis in immunocompromised adults with acquired immunodeficiency syndrome (AIDS), advanced AIDS-related complex (ARC), malignancy, or other severe underlying disease and is also used orally or IV for the treatment of esophageal candidiasis in adults with AIDS, malignancy, or other severe underlying disease, including progressive systemic sclerosis.
Fluconazole appears to be at least as effective as and, in some cases, more effective than other antifungal agents used in the initial treatment of oropharyngeal and/or esophageal candidal infections. It is considered a drug of choice for these infections.
Fluconazole has produced clinical resolution of signs and symptoms of the infection in 79-100% of patients with oropharyngeal candidiasis; however, microbiologic cures generally have been obtained in 44-87% of patients, and the rate of relapse may be high, especially in neutropenic patients. In adults with esophageal candidiasis documented by endoscopy, fluconazole has produced clinical resolution of signs and symptoms of the infection in about 61-93% of patients. In one study in adults with esophageal candidiasis and progressive systemic sclerosis, fluconazole therapy produced mycologic cures in about 93% of patients within 2-4 weeks. Still, the relapse rate was almost 100% within 3 months after fluconazole therapy was discontinued.
HIV-infected patients with severe or recurrent episodes of oropharyngeal or esophageal candidiasis may benefit from long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent relapse.
Some clinicians consider topical therapy with oral clotrimazole or oral nystatin the treatment of choice for uncomplicated oropharyngeal candidiasis in HIV-infected individuals and recommend that systemic antifungal agents (e.g., oral fluconazole, oral itraconazole, oral ketoconazole) be reserved for the treatment of oropharyngeal candidiasis unresponsive to topical agents and the treatment of severe oropharyngeal candidiasis with esophageal involvement. However, some clinicians prefer to use an oral azole antifungal agent for initial therapy of oropharyngeal candidiasis.
Oral fluconazole or itraconazole oral solution is at least as effective as topical therapy. In a study of HIV-infected adults with oropharyngeal candidiasis, the response rate and mycologic eradication rate after 14 days of therapy were 100 and 75%, respectively, in those who received oral fluconazole (100 mg once daily) and were 65 and 20%, respectively, in those who received topical clotrimazole (10-mg oral lozenge 5 times daily). In another study, 14 days of therapy with oral fluconazole (100 mg once daily as an oral suspension) was more effective than 14 days with topical nystatin (500,000 units as an oral suspension 4 times daily). The mycologic cure rate was 60% in the fluconazole group and 6% in the nystatin group, and the rate of relapse at day 42 was 27 and 11%, respectively. After long-term follow-up, the comparative efficacy of fluconazole and other antifungal agents needs further study.
Systemic anti-infective therapy is necessary for the treatment of esophageal candidiasis. Some clinicians state that a 14- to 21-day regimen of oral fluconazole (100 mg once daily) or itraconazole oral solution (200 mg once daily) is highly effective for the treatment of esophageal candidiasis, and oral ketoconazole is less effective. These clinicians suggest an oral itraconazole solution should be used in patients who fail to respond to oral fluconazole. IV amphotericin B can be used in patients with otherwise refractory disease. In a randomized, multicenter, double-blind study comparing oral fluconazole (initial dosage 100 mg daily increased to 200 mg daily if no symptomatic improvement occurred within 1-2 weeks) for up to 8 weeks with oral ketoconazole (initial dosage 200 mg daily increased to 400 mg daily if no symptomatic improvement occurred within 1-2 weeks) in AIDS patients with endoscopically confirmed esophageal candidiasis, endoscopic cure and resolution of esophageal symptoms occurred in 91 and 85% of patients receiving fluconazole, respectively, while endoscopic cure and resolution of symptoms occurred in 52 and 65% of patients receiving ketoconazole, respectively; results of another study in immunocompromised patients with esophageal candidiasis indicate that patients receiving fluconazole may require a shorter duration of initial therapy than those receiving ketoconazole.
Vulvovaginal Candidiasis
Oral fluconazole is used for the treatment of uncomplicated vulvovaginal candidiasis and the treatment of complicated vulvovaginal candidiasis in nonpregnant women. Before initial use of antifungal therapy in a woman who has signs and symptoms of uncomplicated vulvovaginal candidiasis, the diagnosis should be confirmed either by demonstrating yeasts or pseudohyphae with direct microscopic examination of vaginal discharge (10% potassium hydroxide [KOH] wet mount or Gram stain) or by culture; identifying Candida by culture in the absence of symptoms is not an indication for antifungal treatment since approximately 10-20% of women harbor Candida or other yeasts in the vagina. In women with recurrent vulvovaginal candidiasis, vaginal cultures should be obtained to confirm the diagnosis and identify unusual Candida species (e.g., C. glabrata).
Uncomplicated Vulvovaginal Candidiasis
Oral fluconazole effectively treats uncomplicated vulvovaginal candidiasis when given as a single dose. A single 150-mg oral dose of fluconazole produces clinical cures (i.e., absence of vulvovaginal burning, itching, swelling, erythema, excoriation, dyspareunia, and/or ulceration and substantial decreases in vaginal discharge) 5-16 days after the dose in approximately 90-100% and mycologic cures in approximately 77-100% of nonpregnant women with uncomplicated vulvovaginal candidiasis. At 27-62 days after the single dose, clinical and mycologic cure rates are 61-90%, and relapse, reinfection, or recolonization rate is about 23%. Results of several studies in patients with uncomplicated vulvovaginal candidiasis suggest that a single 150-mg dose of oral fluconazole is as effective for this condition as multiple-dose regimens of intravaginal clotrimazole, econazole, miconazole, or terconazole. In addition, the single-dose oral fluconazole regimen is as effective for uncomplicated vulvovaginal candidiasis as oral itraconazole or oral ketoconazole.
In controlled studies in patients with vulvovaginal candidiasis, clinical and mycologic cure rates at 14 and 30-35 days were similar in patients receiving oral fluconazole (given as a single 150-mg dose) compared with patients receiving intravaginal clotrimazole (given as a 100-mg vaginal tablet once daily for 7 days) or miconazole (given as a 100-mg vaginal cream once daily for 7 days). At 14 days, the clinical cure rate was reported to be about 95-96% with fluconazole and 95-97% with intravaginal clotrimazole or miconazole, and the mycologic cure rate was reported to be 77-80% with fluconazole and 72-82% with intravaginal clotrimazole or miconazole. At 30-35 days, the clinical cure rate was reported to be about 69-75% with fluconazole and 72-80% with intravaginal clotrimazole or miconazole, and the mycologic cure rate was reported to be 61-63% with fluconazole, and 57-63% with intravaginal clotrimazole or miconazole.
The US Centers for Disease Control and Prevention (CDC) and other clinicians recommend that uncomplicated vulvovaginal candidiasis (defined as vulvovaginal candidiasis that is mild to moderate, sporadic or infrequent, most likely caused by C. albicans, or occurring in immunocompetent women) be treated with an intravaginal azole antifungal (e.g., butoconazole, clotrimazole, miconazole, terconazole, tioconazole) given inappropriate single-dose or short-course regimens or oral fluconazole given in a single-dose regimen. These regimens generally have been associated with clinical and mycologic cure rates of 80-90% in otherwise healthy, nonpregnant women with uncomplicated infections. Some clinicians suggest that a single oral dose of fluconazole may offer some advantage over conventional intravaginal therapy since it ensures compliance and may reduce or eliminate concurrent rectal infections that may serve as a source of reinfection. In weighing the potential risks and benefits of oral versus intravaginal therapy, the potential for toxicity (e.g., hepatotoxicity) and drug interactions associated with oral therapy should be considered. The incidence of adverse effects is higher in patients receiving single oral doses of fluconazole compared with those receiving intravaginal therapy, and this also should be weighed carefully.
Complicated and Recurrent Vulvovaginal Candidiasis
Oral fluconazole treats complicated vulvovaginal candidiasis, including recurrent and severe infections. Complicated vulvovaginal candidiasis is defined as infections that are recurrent or severe, caused by Candida other than C. albicans, or occurring in pregnant women or women with underlying diseases such as uncontrolled diabetes, debilitation, or immunosuppression.
Optimum regimens for treating recurrent vulvovaginal candidiasis (usually defined as 4 or more episodes of symptomatic vulvovaginal candidiasis each year) have not been established. Although each episode caused by C. albicans may respond to usual single-dose oral fluconazole or short-course intravaginal antifungal therapy, a longer duration of initial therapy may be necessary to achieve mycologic remission and chronic maintenance therapy may be necessary to prevent relapse. The CDC and other clinicians recommend the use of an initial intensive regimen consisting of 7-14 days of an intravaginal azole antifungal or a 2-dose regimen of oral fluconazole (150 mg repeated 3 days later) followed by a maintenance antifungal regimen (given for 6 months). For the maintenance regimen, the CDC recommends intravaginal clotrimazole (500 mg once weekly), oral ketoconazole (100 mg once daily), oral fluconazole (100-150 mg once weekly), or oral itraconazole (400 mg once monthly or 100 mg once daily). These maintenance regimens can effectively reduce recurrent infections; however, 30-40% of women will have recurrent disease once maintenance therapy is discontinued.
The response rate to short-course antifungal regimens is lower in patients with severe vulvovaginal candidiasis (i.e., extensive vulvar erythema, edema, excoriation, and fissure formation). Either a 2-dose regimen of oral fluconazole (150 mg repeated 3 days later) or 7-14 days of an intravaginal azole antifungal is recommended for these infections. These more prolonged regimens may also be necessary for the treatment of vulvovaginal candidiasis in women with underlying debilitating medical conditions (e.g., those with uncontrolled diabetes or those receiving corticosteroid therapy). v Vulvovaginal candidiasis may occur more frequently and may be more severe in women with human immunodeficiency virus (HIV) infection than in women without HIV infection. These infections have been recognized as an early manifestation of acquired immunodeficiency syndrome (AIDS) in women. While optimum therapy for recurrent vulvovaginal candidiasis in HIV-infected women has not been established, there is no evidence to date that these women have a lower response rate to the intravaginal or oral antifungal regimens usually recommended for the treatment of vulvovaginal candidiasis. Therefore, the CDC and other clinicians recommend that the treatment of vulvovaginal candidiasis in HIV-infected women should be the same as that in women without HIV infection.
Recurrent vulvovaginal candidiasis rarely may be caused by resistant strains of C. albicans or, more commonly, by other Candida with reduced susceptibility to azole antifungal agents (e.g., C. glabrata). It has been suggested that repeated treatment of recurrent vulvovaginal candidiasis with intravaginal azole antifungal agents and widespread and/or injudicious use of these agents for self-medication of vulvovaginal candidiasis may favor the selection of Candida that are resistant to azole antifungal agents. Optimum therapy for treating vulvovaginal candidiasis caused by Candida with reduced susceptibility to azole antifungal agents has not been determined. For the treatment of vulvovaginal candidiasis caused by Candida other than C. albicans, the CDC recommends 7-14 days of therapy with an antifungal agent other than fluconazole; if recurrence occurs, intravaginal boric acid (600-mg capsule once daily for 2 weeks) is recommended. Referral to a specialist is advised.
Candidemia and Other Candidal Infections
Fluconazole has effectively treated serious candidal urinary tract infections, peritonitis, and pneumonia. The drug has also been effective in the treatment of chronic mucocutaneous candidiasis, candidemia, chronic disseminated candidiasis (hepatosplenic candidiasis), candidal endocarditis, candidal meningitis, candidal osteomyelitis, and other severe systemic candidal infections. The drug has been effective in treating some candidal infections that did not respond to therapy with amphotericin B. Fluconazole has been effective in treating life-threatening candidal infections in organ transplant patients receiving immunosuppressive therapy. In some renal allograft recipients, fluconazole therapy effectively eliminated fungal infections without discontinuing or decreasing the dosage of immunosuppressive therapy.
Both IV amphotericin B and IV or oral fluconazole are considered drugs of choice for systemic invasive candidiasis; however, optimal antifungal regimens for these infections have been difficult to identify, and drawbacks are associated with each agent. While fluconazole may be better tolerated and easier to administer than IV amphotericin B, fluconazole-resistant strains of C. albicans are being isolated with increasing frequency from patients who have received prior fluconazole therapy (especially in HIV-infected patients). Some candidal infections (e.g., candidemia) are increasingly caused by candidal strains that are intrinsically resistant to fluconazole (e.g., C. krusei) or likely to be fluconazole-resistant (e.g., C. glabrata). The choice of antifungal agent for the initial treatment of invasive candidal infections should take into consideration local and/or institutional epidemiologic data regarding the prevalence of the various candidal strains and their patterns of resistance, the colonization status of the patient, severity, and duration of neutropenia or immunosuppression, and history relating to prior fluconazole use. Most clinicians recommend IV amphotericin B if the infecting organism is known or likely to be C. krusei; however, fluconazole is preferred if C. lusitaniae causes the infection.
Because candidemia is associated with substantial morbidity and risk of long-term sequelae, antifungal agent therapy is generally recommended for all patients with candidemia (regardless of whether they are neutropenic or nonneutropenic) in addition to removal and/or exchange of any intravascular catheters. It has been suggested that oral fluconazole may be preferred over IV amphotericin B for the treatment of candidemia in nonneutropenic patients (both stable and unstable patients) unless there is evidence that the infection is caused by fluconazole-resistant strains or the patient previously received fluconazole. IV amphotericin B generally is preferred for the treatment of severe candidemia in patients who have infections caused by strains that may be fluconazole-resistant (e.g., C. krusei, C. glabrata), patients who have recently received fluconazole, and immunocompromised patients such as those with HIV infection. In a controlled study in patients with candidemia who were immunocompetent and had normal neutrophil counts, the response to conventional IV amphotericin B therapy (0.-0. mg/kg daily for 7 days, then 3 times weekly for an additional 10 days) or fluconazole therapy (400 mg daily given IV for 7 days, then orally for an additional 11 days) was similar. Both regimens appeared to be equally effective. The overall response rate to a conventional IV amphotericin B regimen or oral fluconazole was also similar in a prospective, randomized study that included nonneutropenic and neutropenic patients with documented or presumed invasive candidiasis. However, further study is needed to more fully evaluate the relative efficacy of amphotericin B and fluconazole in immunocompromised patients or patients with severe infections.
Oral fluconazole or IV amphotericin B are recommended when treatment of candiduria is indicated (e.g., symptomatic or neutropenic patients, low-birthweight infants, patients with renal allografts, patients who will undergo urologic manipulations); fluconazole may be preferred unless C. krusei or C. glabrata causes the infection. Bladder irrigation with conventional amphotericin B also has been used. In a randomized study in hospitalized, geriatric patients with fungi caused by C. albicans, C. tropicalis, or C. glabrata, 5 days of bladder irrigation with conventional amphotericin B (25 mg of amphotericin B in 500 mL of 5% dextrose injection infused through an indwelling bladder catheter at a rate of 42 mL/hour) or a 5-day course of oral fluconazole (200-mg loading dose on day 1 followed by 100 mg once daily for four additional days) eradicated the funguria in 83 or 66% of patients, respectively.
Fluconazole is also used prophylactically to reduce the incidence of candidiasis in bone marrow transplant recipients who are receiving chemotherapy or radiation therapy. The drug has also been used for primary prophylaxis against fungal infections, including candidiasis, in a limited number of patients considered at high risk for developing such infections (e.g., neutropenic cancer patients, colonized with Candida and/or are receiving corticosteroids and certain AIDS patients).
Fluconazole has been used with good results in several patients with endophthalmitis caused by Candida. Still, treatment failures have been reported, and the role of the drug in the treatment of this infection remains to be elucidated. Studies in rabbits indicate that fluconazole is distributed into the eye and that the drug inhibits the growth of C. albicans in rabbit choroid-retina tissue and vitreous body when IV therapy is initiated within 24 hours postinoculation; the drug did not effectively inhibit the growth of the organism when IV therapy was initiated 7 days postinoculation when the infection was well established.
Cryptococcal Infections
Oral or IV fluconazole is used in immunocompetent or immunocompromised adults to treat meningitis caused by C. neoformans. The drug has been effective for the initial (primary) treatment of acute cryptococcal meningitis in both HIV-infected and HIV-negative adults. It has produced clinical resolution of signs and symptoms of the infection in approximately 34-75% of these patients. Although amphotericin B (with or without concomitant flucytosine) has been considered the initial treatment of choice for cryptococcal meningitis, fluconazole is an alternative for these infections in patients whose disease is not severe since it generally is well tolerated and is distributed into CSF in high concentrations. While experience in children is limited, fluconazole also may be considered as an alternative to amphotericin B therapy in this age group.
Fluconazole has been effective in the treatment of acute cryptococcal meningitis in some patients who failed to respond to amphotericin B therapy. However, there is some evidence that fluconazole may be less effective than amphotericin B during early therapy of acute cryptococcal meningitis in patients with AIDS and may produce slower sterilization of CSF. In a randomized, multicenter study comparing amphotericin B (mean dosage of 0.4-0.5 mg/kg daily for 10 weeks with or without concomitant flucytosine) with oral fluconazole (400 mg on the first day and 200-400 mg thereafter for 10 weeks) in AIDS patients with cryptococcal meningitis, therapy was effective in 40% of patients receiving amphotericin B and in 34% of those receiving fluconazole. Although overall mortality between patients receiving amphotericin B and patients receiving fluconazole was similar (14% in patients receiving amphotericin B versus 18% in patients receiving fluconazole), mortality was higher during the first 2 weeks of therapy in patients receiving fluconazole (15% versus 8% in those receiving amphotericin B). CSF cultures were positive for an average of about 42 or 64 days in patients receiving amphotericin B or fluconazole, respectively. In another study comparing amphotericin B (0.7 mg/kg daily for 1 week, followed by this dose 3 times weekly for 9 weeks combined with flucytosine 150 mg/kg daily) with oral fluconazole (400 mg daily for 10 weeks) in a limited number of AIDS patients with cryptococcal meningitis, initial therapy was effective in all patients receiving amphotericin B but in only 43% of patients receiving fluconazole; CSF cultures were positive for an average of about 16 and 41 days in patients receiving these respective therapies. While patient groups in this study were similar concerning the severity of cryptococcal infection, the helper/inducer (CD4+, T4+) T-cell count was lower in the fluconazole group, confounding interpretation.
Many clinicians recommend that treatment of cryptococcal meningitis in HIV-infected patients be initiated with a regimen of IV amphotericin B (with flucytosine) given for at least 2 weeks (or until the patient’s condition is stabilized) followed by a regimen of oral fluconazole or oral itraconazole administered for at least an additional 8-10 weeks or longer. Pending further accumulation of data and experience, some clinicians state that initial (primary) therapy with IV amphotericin B (with or without flucytosine) followed by fluconazole maintenance therapy may be most prudent, at least in patients with more severe disease and those at high risk, and that primary fluconazole therapy probably should be reserved for patients failing to respond to or intolerant of amphotericin B adequately and those with less severe disease (e.g., absence of neurologic manifestations, low CSF cryptococcal antigen titers).
The relative efficacy of initial therapy with conventional IV amphotericin B (0. mg/kg daily) given with flucytosine (100 mg/kg daily) or placebo for 2 weeks followed by oral fluconazole (800 mg daily for 2 days, then 400 mg daily for 8 weeks) or oral itraconazole (600 mg daily for 3 days, then 400 mg daily for 8 weeks) has been evaluated in a double-blind multicenter trial in patients with AIDS-associated cryptococcal meningitis. At 2 weeks, CSF cultures were negative in 60% of those who received amphotericin B with flucytosine compared with 51% of those who received amphotericin B alone. The clinical response to oral fluconazole or oral itraconazole for follow-up therapy was similar. Still, the rate of CSF sterilization at 10 weeks was higher in those who received fluconazole (72%) than those who received itraconazole (60%).
A regimen of oral fluconazole (400 mg daily for pulmonary infections or 400-800 mg daily for CNS infections) and oral flucytosine (100-150 mg/kg daily) has been used as an alternative regimen for the treatment of pulmonary or CNS cryptococcal infections in a limited number of patients. Although fluconazole used with flucytosine may be effective for treating mild to moderate pulmonary cryptococcal infections, this regimen has been ineffective in some patients with cryptococcal meningitis. It is not recommended for initial treatment of such infections. In addition, when used for the treatment of cryptococcal meningitis in HIV-infected individuals, a regimen of fluconazole and flucytosine has been associated with a high incidence of adverse effects resulting in discontinuance of flucytosine in 28% of patients.
The mortality rate in AIDS patients during the first episode of cryptococcal meningitis has been about 25-58% despite amphotericin B therapy. In patients with AIDS who respond to initial antifungal therapy, the rate of relapse of cryptococcal infection is 35-65%. Chronic maintenance or suppressive antifungal therapy is generally considered necessary after initial infection treatment unless immune recovery has occurred due to potent antiretroviral therapy.
Oral fluconazole has been effective when used for long-term maintenance therapy for the prevention of relapse of cryptococcal meningitis in patients with AIDS. (See Uses: Prevention of Fungal Infections in HIV-infected Individuals.) Results of a multicenter study comparing safety and efficacy of oral fluconazole (200 mg once daily) with those of IV amphotericin B (1 mg/kg once weekly) for prevention of relapse of the disease in AIDS patients who have negative cryptococcal cultures after initial adequate amphotericin B therapy indicate that the fluconazole regimen is more effective (in terms of preventing relapse of culture-positive meningitis) and better tolerated than the amphotericin B regimen for maintenance therapy in these patients. In a multicenter study comparing oral fluconazole maintenance (100-200 mg daily) with placebo in such patients, the overall calculated cumulative risk of cryptococcal recurrence at any site after 1 year of fluconazole maintenance was 5% versus 100% for placebo.
The efficacy of fluconazole maintenance in preventing cryptococcal relapse in patients with persistent prostatic infection following primary antifungal therapy appears to be lower than that in patients overall. Still, such reduced efficacy may be overcome at least partially by increasing the dosage of the drug. In a limited number of adults with AIDS who had persistent urinary cryptococcosis (including prostate infections) following adequate primary amphotericin B therapy (with or without flucytosine) for cryptococcal meningitis, oral fluconazole maintenance therapy resulted in mycologic cures in most patients. In contrast, relapse of cryptococcosis (with prostatic massage) and/or systemic infection occurred in the remaining patients during fluconazole maintenance.
Response (sustained suppression of prostatic massage-induced cryptococcus and absence of evidence of systemic or CNS relapse) in such patients appears to be dose-dependent, generally requiring oral fluconazole dosages of 200-600 mg daily, and requires several weeks to months of fluconazole maintenance to become manifest (in one study, the probability of response was estimated to be 36% after 4 weeks and 59% after 27 weeks of maintenance). Relatively large dosages (e.g., 600 mg or more daily) may effectively sterilize multiple large prostatic abscesses in some patients. However, factors that may predict response and optimum antifungal therapy in such patients remain to be more fully elucidated. In addition, because of the risk of relapse, some clinicians caution that the possibility of a prostatic focus of infection should be adequately excluded in any male patient if low-dose fluconazole maintenance is contemplated.
Oral fluconazole has been effective in a limited number of patients for treating cutaneous or subcutaneous cryptococcosis. The drug has also been effective for treating cryptococcal pneumonia in a limited number of patients, although surgical removal of the focus of infection was necessary for some patients; however, the possibility that cryptococcal pneumonia may be a manifestation of disseminated infection should be considered.
Coccidioidomycosis
Oral fluconazole has been used successfully in treating coccidioidomycosis caused by Coccidioides immitis (e.g., meningitis, pulmonary infections, disseminated infections including soft tissue or bone and joint). In adults with coccidioidal meningitis, fluconazole has produced clinical and/or laboratory evidence of improvement when used alone or in conjunction with amphotericin B therapy. Oral fluconazole has been used for the treatment of coccidioidal meningitis in both HIV-infected and HIV-negative individuals. Because fluconazole generally is well tolerated and exhibits favorable pharmacokinetics (e.g., is distributed into CSF in high concentrations following oral or IV administration), the drug is considered a less toxic alternative to amphotericin B for the treatment of coccidioidal meningitis and other persistent coccidioidal infections, especially since long-term antifungal therapy usually is necessary for these infections. Fluconazole is considered a drug of choice for the treatment of coccidioidomycosis; however, IV amphotericin B is generally preferred for the initial treatment of severe coccidioidomycosis, especially in immunocompromised patients, including HIV-infected individuals. Further study is needed to evaluate the efficacy and establish the optimum oral fluconazole dosage for coccidioidomycosis and determine whether the drug substantially reduces morbidity and mortality. Some data indicate that the response rate may be improved when higher fluconazole dosages (i.e., 400 mg daily or higher) are used. It has been suggested that using relatively low dosages (e.g., 50-100 mg daily) may contribute to the risk of infection recurrence.
Blastomycosis
Oral fluconazole has been used to treat North American blastomycosis caused by Blastomyces dermatitidis. While oral itraconazole or IV amphotericin B are considered drugs of choice for the treatment of blastomycosis, oral fluconazole or oral ketoconazole are considered alternative agents. IV amphotericin B is generally preferred for the treatment of severe infections, especially those involving the CNS, and for the initial treatment of presumptive blastomycosis in immunocompromised patients, including HIV-infected individuals.
There is some evidence that oral itraconazole may be more effective than oral fluconazole or oral ketoconazole for treating blastomycosis. Many clinicians consider itraconazole the preferred azole antifungal agent for the treatment of nonmeningeal, non-life-threatening blastomycosis and also recommend the drug for follow-up therapy in patients with more severe infections after an initial response has been obtained with IV amphotericin B. The fact that treatment failures have been reported when an oral antifungal agent (e.g., ketoconazole) was used in the treatment of cutaneous or pulmonary blastomycosis in patients who had asymptomatic or subclinical CNS involvement at the time of the initial diagnosis should be considered when selecting an antifungal agent for patients with blastomycosis.
Histoplasmosis
Oral fluconazole has been used with some success for the treatment of histoplasmosis caused by Histoplasma capsulatum. While IV amphotericin B or oral itraconazole are considered drugs of choice for the treatment of histoplasmosis, oral fluconazole or oral ketoconazole are considered alternative agents. Oral fluconazole (400-800 mg daily) has been effective when used in a limited number of HIV-infected patients with mild or moderately severe disseminated histoplasmosis; however, IV amphotericin B is generally preferred for the initial treatment of severe, life-threatening histoplasmosis, especially in immunocompromised patients such as those with HIV infection. In addition, oral itraconazole is generally the preferred azole antifungal agent for treating mild to moderate histoplasmosis or as follow-up therapy in treating severe infections after a response has been obtained with amphotericin B.
Sporotrichosis
Fluconazole is used as an alternative agent for the treatment of sporotrichosis. IV amphotericin B usually is considered the drug of choice for the initial treatment of severe, life-threatening infections, and whenever there is CNS involvement and oral itraconazole is considered the drug of choice for the treatment of cutaneous, lymphocutaneous, or mild pulmonary or osteoarticular sporotrichosis and for follow-up therapy in more severe infections after a response has been obtained with IV amphotericin B. Fluconazole is considered second-line therapy for the treatment of cutaneous, lymphocutaneous, or osteoarticular sporotrichosis and, because it may be less effective than itraconazole, should be used be used only if the patient cannot tolerate itraconazole. Some clinicians state that fluconazole is not effective and should not be used for the treatment of pulmonary sporotrichosis.
Aspergillosis
Fluconazole has been used orally, IV, or by intracavitary infusion in a few adults for the treatment of pneumonia or other respiratory tract infections caused by Aspergillus fumigatus, A. niger, or A. terreus. In one study, fluconazole had a 23-53% clinical efficacy rate in Aspergillus infections and a mycologic cure rate of about 50% for A. fumigatus infections. Fluconazole has produced variable results in treating infections caused by Aspergillus. IV amphotericin B is generally considered the drug of choice, and itraconazole is usually the preferred alternative for treating aspergillosis.
Dermatophytoses
Oral fluconazole has been effective when used in the treatment of certain dermatophytoses (e.g., tinea capitis, tinea corporis, tinea cruris, tinea pedis) caused by Epidermophyton, Microsporum, or Trichophyton. Oral fluconazole has also been effective for treating pityriasis (tinea) versicolor and onychomycosis.
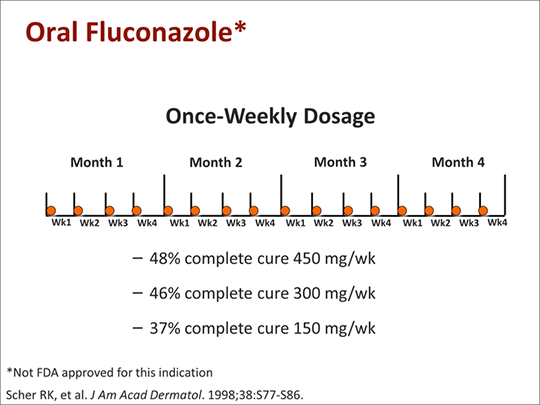
Oral fluconazole (3-6 mg/kg daily for 2-6 weeks) has effectively treated tinea capitis in children 1.5-16 years of age and has resulted in a clinical and mycologic cure in about 88-90% of patients. For treating tinea corporis, tinea cruris, or tinea pedis in adults, oral fluconazole has been effective in a once-weekly regimen (150 mg once weekly for 2-6 weeks). There is evidence that this once-weekly regimen is as effective as a once-daily regimen of the drug (50 mg once daily) for treating these infections. Results of a randomized study indicate that the eradication rate at the end of treatment in patients with tinea corporis or tinea cruris is 82-88% in those receiving the once-weekly regimen or 94-100% in those receiving the once-daily regimen; at 1-month follow-up, the overall eradication rates were 91-100 or 91-94%, respectively. Although the optimum dosage regimen of oral fluconazole for the treatment of onychomycosis has not been identified, a once-weekly oral fluconazole regimen (150-450 mg once weekly for 3-12 months) has been effective for the treatment of onychomycosis of the toenail in a limited number of adults. However, additional study is needed, and there is some evidence that fluconazole may be less effective than oral itraconazole or oral terbinafine for treating onychomycosis.
Tinea corporis and tinea cruris generally can be effectively treated using a topical antifungal agent; however, an oral antifungal regimen may be necessary if the disease is extensive, dermatophyte folliculitis is present, the infection is chronic or does not respond to topical therapy, or the patient is immunocompromised or has coexisting disease. Tinea capitis and tinea barbae are generally treated using an oral antifungal regimen. While topical antifungals are usually adequate for the treatment of uncomplicated tinea manuum and tinea pedis, an oral antifungal regimen is usually necessary for the treatment of hyperkeratotic areas of the palms and soles, for the treatment of chronic moccasin-type (dry-type) tinea pedis, and for the treatment of tinea unguium (onychomycosis).
Prevention of Fungal Infections in HIV-infected Individuals
Fluconazole has been used in HIV-infected individuals for primary prophylaxis against serious fungal infections (e.g., cryptococcosis) and for long-term suppressive or chronic maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of certain fungal infections (e.g., coccidioidomycosis, cryptococcosis, mucocutaneous candidiasis).
The Prevention of Opportunistic Infections Working Group of the US Public Health Service and the Infectious Diseases Society of America (USPHS/IDSA) has established guidelines for the prevention of opportunistic infections, including fungal infections, in HIV-infected individuals that include recommendations concerning prevention of exposure to opportunistic pathogens, prevention of first disease episodes, and prevention of disease recurrence. The USPHS/IDSA states that primary prophylaxis to prevent first episodes of mucocutaneous candidiasis in HIV-infected adults, adolescents, infants, and children is not recommended. While routine primary prophylaxis to prevent first episodes of coccidioidomycosis, cryptococcosis, or histoplasmosis in HIV-infected adults, adolescents, infants, and children is not recommended, the USPHS/IDSA states that primary prophylaxis against cryptococcosis or histoplasmosis may be considered in specifically selected individuals. The USPHS/IDSA recommends that HIV-infected adults, adolescents, infants, and children who have completed initial therapy for documented coccidioidomycosis, cryptococcosis, or histoplasmosis receive long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of these fungal infections. In addition, the USPHS/IDSA states that HIV-infected individuals who have frequent or severe recurrences of mucocutaneous candidiasis (oropharyngeal, esophageal, vaginal) may benefit from long-term suppressive or maintenance therapy.
Because of concerns regarding oral azole antifungal agent use during pregnancy, fluconazole should not be used for primary prophylaxis or chronic suppressive or maintenance therapy in pregnant women. If a woman becomes pregnant while receiving fluconazole prophylaxis and elects to continue the pregnancy, prophylaxis should be discontinued. Effective contraceptive measures are recommended for all HIV-infected women receiving an oral azole antifungal agent for suppressive therapy. Conventional IV amphotericin B may be the preferred agent if long-term suppressive or maintenance therapy against coccidioidomycosis, cryptococcosis, or histoplasmosis is indicated in an HIV-infected pregnant woman, especially during the first trimester.
Primary Prophylaxis
Coccidioidomycosis
The USPHS/IDSA states that routine primary prophylaxis against coccidioidomycosis in HIV-infected patients living in coccidioidomycosis-endemic areas is not recommended. Some clinicians suggest that primary prophylaxis should be considered for HIV-infected individuals who have positive CF serologic results without active disease. Still, it is unclear whether primary prophylaxis would be helpful for HIV-infected individuals living in areas endemic to coccidioidomycosis. Active coccidioidomycosis has developed in HIV-infected individuals who were receiving azole therapy for other conditions. Routine skin testing with coccidiosis in HIV-infected individuals who live in areas endemic for coccidioidomycosis is not predictive of disease and generally is not recommended. Within an endemic area, a positive serologic test might indicate an increased risk for active infection; however, routine serologic testing does not appear helpful or recommended.
Cryptococcosis
The USPHS/IDSA states that, although routine primary prophylaxis against cryptococcosis is not recommended, primary prophylaxis may be considered in HIV-infected adults and adolescents with CD4+ T-cell counts less than 50/mm3 and infants and children with severe immunosuppression (as defined by age-adjusted criteria). Routine prophylaxis is not recommended because of the relative infrequency of cryptococcal disease, lack of evidence of survival benefit associated with prophylaxis, and concerns regarding the possibility of drug interactions, potential for development of resistance, and cost. The need for primary prophylaxis or suppressive therapy against other fungal infections (e.g., coccidioidomycosis, histoplasmosis, mucocutaneous candidiasis) should be considered while deciding on primary prophylaxis against cryptococcosis. Routine testing of asymptomatic individuals for serum cryptococcal antigen is not recommended because of the low probability that results will affect clinical decisions. HIV-infected individuals cannot completely avoid exposure to Cryptococcus neoformans; there is no evidence that exposure to pigeon droppings is associated with an increased risk for cryptococcosis.
Oral fluconazole is the agent of choice for primary prophylaxis against cryptococcosis in HIV-infected adults, adolescents, infants, and children, and itraconazole (given as oral capsules) is considered an alternative.
Histoplasmosis
The USPHS/IDSA states that primary prophylaxis against histoplasmosis may be considered in HIV-infected adults or adolescents with CD4+ T-cell counts less than 100/mm3 who are at especially high risk of exposure to Histoplasma capsulatum because of occupational exposure or who live in a community with a hyperendemic rate of histoplasmosis (at least 10 cases/100 patient-years) and also may be considered for HIV-infected infants or children with severe immunosuppression who live in areas endemic for histoplasmosis. When a decision is being made regarding whether to use primary prophylaxis against histoplasmosis in these HIV-infected individuals, clinicians should consider the local incidence of histoplasmosis, the possibility of drug interactions, toxicity, development of resistance, cost, and the need for prophylaxis against other fungal infections (e.g., candidiasis, cryptococcosis).
The agent of first choice for primary prophylaxis against histoplasmosis in HIV-infected adults, adolescents, or pediatric patients is oral itraconazole (given as capsules); the USPHS/IDSA makes no recommendation regarding an alternative to itraconazole. There is some evidence that oral fluconazole may be ineffective in preventing the first episodes of histoplasmosis.
Mucocutaneous Candidiasis
The USPHS/IDSA states that primary prophylaxis to prevent first episodes of mucocutaneous candidiasis (esophageal, oropharyngeal, vaginal) in HIV-infected adults, adolescents, infants, or children is not recommended because acute mucocutaneous candidiasis generally is treatable and rarely life-threatening and because of concerns about the potential for development of resistant Candida, the possibility of drug interactions, and cost of antifungal prophylaxis.
Prevention of Recurrence
Coccidioidomycosis
The USPHS/IDSA recommends that HIV-infected individuals who have completed initial therapy for documented coccidioidomycosis should receive long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse. The USPHS/IDSA recommends oral fluconazole as the drug of choice and IV amphotericin B and oral itraconazole (given as capsules) as alternatives. Long-term suppressive therapy for prophylaxis against recurrence or relapse of coccidioidomycosis in HIV-infected adults, adolescents, infants, and children generally is continued for life. Although HIV-infected individuals may be at low risk for recurrence of systemic fungal infections if their CD4+ T-cell count increases to greater than 100/mm3 while receiving potent combination antiretroviral therapy, the USPHS/IDSA states that data are insufficient to date to warrant a recommendation regarding discontinuance of prophylaxis against coccidioidomycosis in these individuals.
Cryptococcosis
The USPHS/IDSA recommends that HIV-infected individuals who have completed initial therapy for documented cryptococcosis should receive long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse unless immune recovery has occurred as a result of potent combination antiretroviral therapy. The USPHS/IDSA recommends oral fluconazole as the drug of choice and IV amphotericin B and oral itraconazole (given as capsules) as alternatives. There is some evidence from a randomized, double-blind, controlled study in HIV-infected individuals with documented, adequately treated cryptococcal meningitis that oral fluconazole (200 mg once daily) is more effective than oral itraconazole (200 mg once daily) for suppressive therapy in these patients since the rate of culture-positive relapse of cryptococcosis was 4% in those receiving fluconazole compared with 23% in those receiving itraconazole.

Suppressive or maintenance therapy to prevent recurrence or relapse of cryptococcosis in HIV-infected individuals generally is continued for life unless immune recovery has occurred as a result of potent combination antiretroviral therapy. Limited data indicate that discontinuing suppressive or maintenance therapy in HIV-infected adults and adolescents who have completed initial therapy for cryptococcosis, remain asymptomatic concerning cryptococcosis, and have sustained (e.g., for 6 months or longer) increases in CD4+ T-cell counts to greater than 100-200/mm3 in response to potent antiretroviral therapy is associated with a low risk for recurrence of cryptococcosis. Based on this and more extensive cumulative data on the safety of discontinuing long-term suppressive therapy for other opportunistic infections, the USPHS/IDSA states that it is reasonable to consider discontinuing suppressive therapy in individuals meeting these criteria. The USPHS/IDSA notes that recurrences could occur in individuals discontinuing suppressive therapy and states that suppressive therapy should be restarted if the CD4+ T-cell count decreases to less than 100-200/mm3. The safety of discontinuing suppressive therapy in HIV-infected infants and children has not been studied, and children should receive lifelong suppressive therapy after an episode of cryptococcosis.
Histoplasmosis
For long-term suppressive therapy in HIV-infected patients with documented histoplasmosis that has been adequately treated, the USPHS/IDSA states that oral itraconazole (given as capsules) is the drug of choice and IV amphotericin B is an alternative.
Mucocutaneous Candidiasis
The USPHS/IDSA states that the use of long-term suppressive or maintenance therapy should be considered in adults and adolescents who have a history of documented esophageal candidiasis (especially those who have had multiple episodes), taking into consideration the potential for development of resistant strains of Candida. In addition, the USPHS/IDSA states that the use of suppressive therapy should be considered for infants and children who have severe, recurrent mucocutaneous candidiasis, especially those with esophageal candidiasis. Although many experts do not recommend long-term prophylaxis against recurrent oropharyngeal or vulvovaginal candidiasis in HIV-infected patients for the same reasons they do not recommend routine primary prophylaxis against candidiasis, the USPHS/IDSA states that suppressive therapy may be considered for HIV-infected individuals who have frequent or severe recurrences of these candidal infections. However, several factors should be addressed when considering such therapy, including the impact of recurrences on the patient’s well-being and quality of life, the need for prophylaxis against other fungal infections, the cost of prophylaxis, drug toxicities, drug interactions, and the potential for development of drug resistance among Candida and other fungi.
If long-term suppressive therapy as prophylaxis against mucocutaneous candidiasis is indicated in HIV-infected adults, adolescents, infants, or children with frequent or severe recurrences of oropharyngeal, esophageal, or vaginal candidiasis, the USPHS/IDSA recommends oral fluconazole as the drug of choice and itraconazole (given as the oral solution) as an alternative.
Long-term suppressive therapy for prophylaxis against recurrence or relapse of fungal infections in HIV-infected patients generally is continued for life. In some HIV-infected patients who had been receiving oral fluconazole for prevention of recurrence of oropharyngeal candidiasis for a median duration of 18 months (range: 4-98 months) and were receiving potent combination antiretroviral agent therapy (about 50% had plasma HIV-1 RNA levels lower than the limits of detection), discontinuance of oral fluconazole suppressive therapy resulted in a recurrence of oropharyngeal candidiasis in only 10% of patients within 6-11 months. Although HIV-infected patients receiving suppressive antifungal prophylaxis may be at low risk for recurrence of fungal infections if their CD4+ T-cell counts increase to greater than 100/mm3 while receiving potent combination antiretroviral agent therapy, the USPHS/IDSA states that data are insufficient to date to warrant a recommendation regarding discontinuance of prophylaxis in these individuals.
Prevention of Fungal Infections in Transplant Patients and Patients with Cancer
Fluconazole is used prophylactically to reduce the incidence of candidiasis in patients undergoing bone marrow transplantation (BMT) who are receiving chemotherapy or radiation therapy. The drug has also been used for prophylaxis of fungal infections in patients undergoing liver transplantation and in cancer patients considered at risk for neutropenia and fungal infections. There is some evidence that fluconazole prophylaxis in transplant and cancer patients can reduce the frequency of oropharyngeal and/or systemic candidiasis during the period before neutrophil recovery. In addition, fluconazole prophylaxis may reduce the need for empiric antifungal agent therapy in such patients. The efficacy of oral fluconazole (400 mg once daily) for prophylaxis against fungal infections in neutropenic patients has been evaluated in a randomized, placebo-controlled study involving 274 cancer patients 18-80 years of age receiving cytotoxic chemotherapy or conditioning therapy for BMT. While the percentage of patients not requiring empiric therapy with IV amphotericin B therapy was similar in both groups (57% of those receiving fluconazole and 50% of those receiving placebo required no such therapy), complete success without fungal colonization was achieved in 37% of those receiving fluconazole and 20% of those receiving placebo. In addition, there was a lower incidence of superficial fungal infections in those receiving fluconazole (7%) than in those receiving placebo (18%), and only 3% of those receiving fluconazole developed definite invasive fungal infections compared with 17% of those receiving placebo. While fluconazole prophylaxis did not affect the overall mortality rate, intent-to-treat analysis indicates that the number of deaths attributable to definite invasive fungal infection was lower in the fluconazole group (1 of 15) than in the placebo group (6 of 15).
The use of primary antifungal prophylaxis in cancer patients undergoing myelosuppressive therapy or patients undergoing BMT or solid organ transplantation remains controversial, particularly since such prophylaxis may predispose the patient to colonization with resistant fungi and/or result in the emergence of highly resistant organisms. Retrospective studies have shown an increased risk of colonization with Candida krusei in BMT recipients and in neutropenic patients who received fluconazole prophylaxis; in one study, about 41% of patients receiving fluconazole had colonization with C. krusei compared with 17% of those not receiving fluconazole. Therefore, most clinicians generally discourage primary prophylactic antifungal therapy, except in certain carefully selected high-risk patients in whom potential benefits are expected to justify possible risks. Many experts, however, state that controlled, randomized studies should continue to evaluate fluconazole’s use to prevent fungal infections in cancer patients and in BMT recipients.
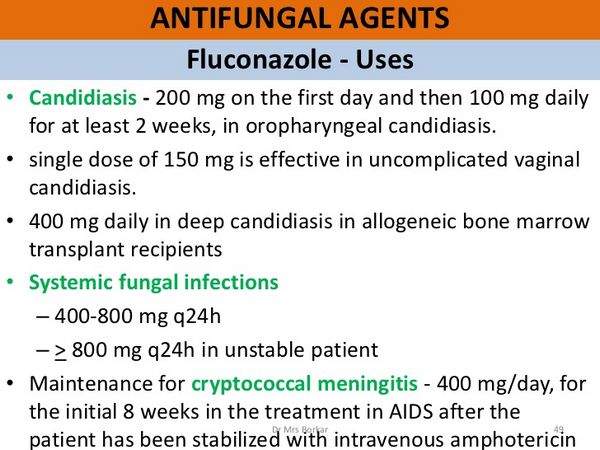
Dosage and Administration
Fluconazole is a triazole antifungal used for superficial mucosal (oropharyngeal, oesophageal, or vaginal) candidiasis and fungal skin infections. It is also given for systemic infections, including systemic candidiasis, coccidioidomycosis, and cryptococcosis. It has been tried in blastomycosis, histoplasmosis, and sporotrichosis.
Fluconazole is given orally or intravenously in similar doses. For intravenous infusion, it is given as a solution containing 2 mg/mL at a rate of 5 to 10 mL/minute (300 to 600 mL/hour). A maximum infusion rate of 100 mL/hour is recommended in the USA.
For superficial mucosal candidiasis (other than genital candidiasis), the usual dose of Fluconazole in the UK is 50 mg daily by mouth. However, 100 mg daily may be given if necessary.
Treatment usually continues for 7 to 14 days in oropharyngeal candidiasis (except in severely immunocompromised patients), for 14 days in atrophic oral candidiasis associated with dentures, and for 14 to 30 days in other mucosal candidal infections, including oesophagitis. Higher doses are recommended in the USA, where an initial dose of Fluconazole 200 mg is followed by 100 mg daily and where the minimum treatment period is 14 days for oropharyngeal infection, or a minimum of 21 days and at least 14 days after resolution of symptoms for oesophageal infections doses of up to 400 mg daily may be used for oesophageal candidiasis if necessary.
Oral Administration
Fluconazole may be given orally without regard to meals. Fluconazole powder for oral suspension should be reconstituted when dispensing by adding 24 mL of distilled or purified water to the container containing 0.35 or 1.4 g of the drug to provide a suspension containing 50 or 200 mg/5 mL, respectively. The bottle should be shaken vigorously to suspend the powder; the suspension should be shaken well just before administration.
IV Administration
IV infusions of Fluconazole should be administered once daily at a rate not exceeding 200 mg/hour. Fluconazole injections for IV infusion should be visually inspected for discoloration and particulate matter before administration whenever solution and container permit. The injection for IV infusion should be discarded if the solution is cloudy or precipitated or if the seal is not intact. Viaflex® Plus containers of Fluconazole should be checked for minute leaks by firmly squeezing the bag. The injection should be discarded if the container seal is not intact, leaks are found, or the solution is cloudy or contains a precipitate. Additives should not be introduced into the plastic injection container. The injection in plastic containers should not be used in series connections with other plastic containers since such use could result in air embolism from residual air being drawn from the primary container before administration from the secondary container is complete.
Fluconazole 150 mg as a single oral dose may be used for genital candidiasis (vaginal candidiasis or candidal balanitis). Dermatophytosis, pityriasis versicolor, and Candida skin infections may be treated with Fluconazole 50 mg taken by mouth daily for up to six weeks.
Systemic candidiasis, cryptococcal meningitis, and other cryptococcal infections may be treated orally or by intravenous infusion with Fluconazole. The initial dose is 400 mg, followed by 200 to 400 mg daily. The duration of therapy is based on clinical and mycological response, but it is usually at least 6 to 8 weeks. In cryptococcal meningitis in the USA, treatment is recommended for 10 to 12 weeks after the CSF cultures become negative.
Fluconazole may also be used in daily doses of 100 to 200 mg orally or intravenously to prevent relapse after a primary course of antifungal treatment for acute cryptococcal meningitis in patients with AIDS. In immunocompromised patients at risk of fungal infections, Fluconazole may be given prophylactically orally or by intravenous infusion at 50 to 400 mg daily. However, long-term prophylaxis has been associated with the emergence of resistant organisms. Doses for children over 4 weeks of age are 3 mg/kg daily for superficial infections (a loading dose of 6 mg/kg may be used on the first day if necessary) and 6 to 12 mg/kg daily for systemic infections. A dose of 3 to 12 mg/kg daily may be given for prophylaxis in immunocompromised children. For infants under 2 weeks of age, all these doses should be given once every 72 hours. For those aged between 2 and 4 weeks, the doses should be given every 48 hours.
A maximum dose of 400 mg daily should not be exceeded in children or 12 mg/kg at appropriate intervals in infants. Dosage may need to be reduced in patients with renal impairment.
High Doses
Patients with life-threatening infections caused by Candida spp., Cryptococcus neoformans, and Coccidioides immitis have been tried at doses higher than those recommended by the licensed product information for Fluconazole.
Dose finding studies have found daily doses of 800 to 1000 mg of Fluconazole to be effective and well-tolerated. In a study of 11 HIV-infected patients who received fluconazole 800 to 1000 mg daily intravenously for 3 weeks, then orally until the CSF culture became negative, 6 patients had responded at 10 weeks, and another 2 improved clinically. Daily doses of up to 800 mg have been used in blastomycosis and coccidioidomycosis, and doses of 10 mg/kg daily have been tried in disseminated candidiasis.
Intermittent Doses
Concern has been expressed about the increasingly widespread use of Fluconazole, particularly the impact of continuous fluconazole therapy in immunocompromised patients on the development of resistance. Nevertheless, Fluconazole remains popular for primary and secondary prophylaxis. Some investigators have suggested intermittent doses’ although this could further increase the risk of infections with resistant organisms. Once-weekly treatment with Fluconazole has been tried in onychomycosis and tinea capitis.
Administration in Renal Impairment
Patients with renal impairment may require dosage reduction. Normal loading or initial doses of Fluconazole should be given on the first day of treatment, and subsequent doses should be adjusted according to creatinine clearance (CC):
- CC more than 50 mL/minute: 100% of the standard recommended dose;
- CC less than 50 mL/minute and not receiving dialysis: 50% of the standard recommended dose;
- patients on regular hemodialysis: 100% of the standard recommended dose after each dialysis session. No dosage adjustment is needed in patients with renal impairment given single-dose therapy.
Adult Dosage
Oropharyngeal and Esophageal Candidiasis
For treating oropharyngeal or esophageal candidiasis, the usual adult dosage of Fluconazole is 200 mg as a single dose on the first day of therapy, followed by 100- or 200-mg doses once daily. Dosages up to 400 mg once daily may be used depending on the patient’s response. Although clinical evidence of oropharyngeal candidiasis generally resolves within several days following initiation of fluconazole therapy, the manufacturer and some clinicians recommend that the drug be continued for at least 2 weeks to decrease the likelihood of relapse.
However, other clinicians question the need for prolonged therapy in patients with this infection. Patients with esophageal candidiasis should receive fluconazole therapy for at least three weeks and at least two weeks after symptoms have resolved. The optimal dosage for maintenance therapy in patients with oropharyngeal candidiasis has not been established. Oral dosages of 50-100 mg once daily have generally been used effectively for maintenance therapy in these patients; dosages up to 200 mg once daily have occasionally been used.
Vulvovaginal Candidiasis
For the treatment of uncomplicated vulvovaginal candidiasis in nonpregnant women, the usual dosage of oral Fluconazole is a single (1 day only) 150-mg oral dose. For the treatment of recurrent vulvovaginal candidiasis in nonpregnant women, two 150-mg doses of oral Fluconazole should be given 3 days apart to achieve mycologic remission. Then, a maintenance regimen of 100-150 mg once weekly should be given for 6 months to prevent recurrence. A 2-dose regimen of oral Fluconazole (two 150-mg doses given 3 days apart) also is recommended for the treatment of severe vulvovaginal candidiasis in nonpregnant women.
Leishmaniasis
Fluconazole has been tried to treat cutaneous leishmaniasis caused by Leishmania major. In a randomized, double-blind, placebo-controlled study, 80 patients received a six-week course of oral Fluconazole 200 mg daily, of whom 63 had complete healing of lesions after 3 months, compared with 22 of 65 patients who received a placebo. However, others have reported a response rate that is not significantly different from the placebo’s.
Other Candidal Infections
For treating systemic candidiasis, the usual adult dosage of Fluconazole is 400 mg as a single dose on the first day of therapy, followed by 200 mg once daily. In a limited number of patients with candidal urinary tract infections and peritonitis, dosages of 50-200 mg daily have been used. The optimum dosage and duration of therapy in patients with candidemia, disseminated candidiasis, and pneumonia have not been established; however, a limited number of such patients have received fluconazole dosages of up to 400 mg daily. Some clinicians have recommended that patients with invasive candidiasis receive Fluconazole in a 400-800 mg dosage daily. Therapy should be continued for at least 4 weeks and at least 2 weeks after symptoms have resolved.
Cryptococcal Infections
To treat cryptococcal meningitis, the usual adult dosage of Fluconazole is 400 mg as a single dose on the first day of therapy, followed by 200- to 400 mg once daily. Some evidence suggests that the 400-mg dosage is more effective than lower dosages in treating this infection. A higher dosage of Fluconazole (i.e., 800-1000 mg daily) has been used in some patients with human immunodeficiency virus (HIV) infection for the treatment of cryptococcal meningitis. For initial therapy of cryptococcal meningitis, Fluconazole usually is continued for 10-12 weeks after the CSF is sterile.

Coccidioidomycosis
For treating coccidioidal meningitis in adults, fluconazole dosages of 200-800 mg once daily have been recommended. For the treatment of coccidioidal meningitis in patients with AIDS, fluconazole dosages of 400-800 mg daily are recommended. Concomitant intracisternal, intraventricular, or intrathecal amphotericin B therapy has been used in some patients.
Blastomycosis or Histoplasmosis
If Fluconazole is used to treat blastomycosis or histoplasmosis, a daily dosage of 400-800 mg is recommended.
Prevention of Fungal Infections in HIV-infected Individuals
For primary prophylaxis against cryptococcosis in adults or adolescents with HIV infection and absolute helper/inducer (CD4+, T4+) T-cell counts less than 50/mm3, the Prevention of Opportunistic Infections Working Group of the US Public Health Service and the Infectious Diseases Society of America (USPHS/IDSA) recommends 100-200 mg of oral Fluconazole once daily.
While there is some evidence that oral Fluconazole given at 400 mg once weekly may be effective for primary prophylaxis against fungal infections in HIV-infected individuals, this regimen is not included in current USPHS/IDSA guidelines. Further study is needed to evaluate the efficacy of regimens other than daily administration for primary prophylaxis.
For long-term suppressive or maintenance therapy (secondary prophylaxis) of coccidioidomycosis in HIV-infected adults or adolescents who have had documented, adequately treated infections, a fluconazole dosage of 400 mg once daily is recommended by the USPHS/IDSA. For suppressive or maintenance therapy to prevent recurrence or relapse of cryptococcosis in HIV-infected adults or adolescents who have had documented, adequately treated infections, the usual dosage of oral Fluconazole is 200 mg once daily.
Some clinicians recommend that, for long-term suppressive therapy against cryptococcosis, oral Fluconazole be given in a dosage of 400 mg daily for the first 4 weeks, followed by 200 mg daily. If oral Fluconazole is used for long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of mucocutaneous candidiasis (oropharyngeal, vaginal, esophageal) in HIV-infected adults or adolescents who have had frequent or severe episodes of these candidal infections, the USPHS/IDSA recommends a dosage of 100-200 mg once daily.
While Fluconazole has been given in a dosage of 200 mg once weekly for long-term suppressive therapy in HIV-infected women with a history of oropharyngeal or vaginal candidiasis, this regimen is not included in current USPHS/IDSA guidelines. There are concerns that such a regimen would promote the emergence of fluconazole-resistant strains of Candida. Long-term suppressive or maintenance therapy for prophylaxis against recurrence or relapse of fungal infections in HIV-infected patients generally is continued for life. However, the USPHS/IDSA states that it may be reasonable to discontinue suppressive or maintenance therapy of cryptococcosis in certain adults and adolescents who have immune recovery as the result of potent combination antiretroviral therapy.
Prevention of Fungal Infections in Transplant Patients and Patients with Cancer
To prevent candidiasis in bone marrow transplant recipients, the recommended dosage of Fluconazole is 400 mg once daily. In patients in whom severe granulocytopenia (neutrophil count less than 500/mm3) is anticipated, fluconazole therapy should be initiated several days before the expected onset of neutropenia. It should be continued for 7 days after the neutrophil count exceeds 1000/mm3.
Pediatric Dosage
The usual dosage of Fluconazole in pediatric patients ranges from 3-12 mg/kg once daily; doses exceeding 600 mg daily are not recommended. The manufacturer states that a dosage of 3, 6, or 12 mg/kg daily in pediatric patients is equivalent to 100, 200, or 400 mg daily, respectively, in adults. Some older children may have clearances similar to those of adults.
To treat meningitis or septicemia caused by susceptible Candida, neonates, and infants 3 months of age or younger have received Fluconazole in a dosage of 5-6 mg/kg once daily, given orally or by IV infusion over 1 hour. In some neonates and infants with septicemia, an initial loading dose of 10 mg/kg was administered, followed by 5 mg/kg once daily. Based on data available regarding the pharmacokinetics of Fluconazole in premature neonates, the manufacturer recommends that neonates 2 weeks of age or younger receive the same daily dose as older children. Still, the dose should be administered once every 72 hours.
Oropharyngeal and Esophageal Candidiasis
For the treatment of oropharyngeal or esophageal candidiasis, the manufacturer recommends that pediatric patients receive 6 mg/kg of Fluconazole on the first day, followed by 3 mg/kg once daily. The dosage for esophageal candidiasis may be increased up to 12 mg/kg daily if necessary, based on the condition of the patient and the response to the drug. Treatment for oropharyngeal candidiasis should be continued for a minimum of 2 weeks to decrease the likelihood of relapse. Treatment of esophageal candidiasis should be continued for at least 3 weeks and at least 2 weeks after symptoms have resolved.
Other Fungal Infections
Fluconazole has been given at a dosage of 6-12 mg/kg daily to treat systemic candidal infections in pediatric patients. The manufacturer recommends treating cryptococcal meningitis in pediatric patients with an initial 12 mg/kg dose on the first day, followed by 6 mg/kg once daily. If necessary, the dosage may be increased to 12 mg/kg daily based on the patient’s condition and response to the drug. Fluconazole should be continued for 10-12 weeks after the CSF becomes culture-negative.
Prevention of Fungal Infections in HIV-infected Individuals
The recommended dosage of oral Fluconazole for primary prophylaxis against cryptococcosis in HIV-infected infants and children with severe immunosuppression is 3-6 mg/kg once daily. If oral Fluconazole is used for long-term suppressive or maintenance therapy to prevent recurrence or relapse of cryptococcosis or mucocutaneous candidiasis (oropharyngeal, esophageal) in HIV-infected infants and children, the recommended dosage is 3-6 mg/kg once daily. A dosage of 6 mg/kg once daily is recommended for prophylaxis against recurrence or relapse of coccidioidomycosis in these pediatric patients.
Interactions
In general, fewer interactions occur with Fluconazole than with Itraconazole or ketoconazole. The use of rifampicin with Fluconazole reduces plasma concentrations of Fluconazole. Hydrochlorothiazide and Fluconazole have resulted in clinically insignificant increases in plasma fluconazole concentrations.
Fluconazole may interfere with the metabolism of some other drugs, mainly through inhibition of the cytochrome P450 isoenzymes CYP3A4 and CYP2C9. This may account for the reported increases in plasma concentrations of bosentan, ciclosporin, midazolam, nevirapine, amitriptyline, nortriptyline, phenytoin, rifabutin, sulfonylurea hypoglycaemics and nateglinide, selective cyclooxygenase-2-inhibitors such as celecoxib and parecoxib, tacrolimus, triazolam, warfarin, and zidovudine fluconazole may inhibit the formation of a toxic metabolite of sulfamethoxazole. Increases in terfenadine concentrations following high doses of Fluconazole have been associated with ECG abnormalities.
A similar effect may be anticipated with astemizole. However, using Fluconazole with cisapride could increase cisapride concentrations and cause associated toxicity. Therefore, it should be avoided. The use of Fluconazole with astemizole, cisapride, or terfenadine should be avoided because of the risk of cardiac arrhythmias. Syncope was attributed to increased amitriptyline concentrations, which occurred when amitriptyline was given with Fluconazole.

Fluconazole may also reduce the clearance of theophylline. The concentration of contraceptive steroids has been reported to be both increased and decreased in patients receiving Fluconazole, and the efficacy of oral contraceptives may be affected. For further information on interactions between drugs metabolized by the cytochrome P450 isoenzyme CYP3 A and azoles, see under Itraconazole.
Fluoroquinolones
Both levofloxacin and fluconazole can prolong the QT interval. The simultaneous use of intravenous levofloxacin and Fluconazole resulted in an episode of torsade de pointes in a patient on hemodialysis.

Microbiological Interactions
A synergistic antifungal effect was seen in vitro with terbinafine and Fluconazole against strains of Candida ahicans.
Resistance
The emergence of strains of Candida spp. resistant to Fluconazole has become increasingly important, particularly in immunocompromised patients receiving long-term prophylaxis with Fluconazole. In addition to resistance in Candida albicans, infections with Candida dubliniensis, Candida glabrata, and Candida krusei, all of which may be less sensitive to Fluconazole than Candida albicans, have been noted in these patients. Secondary resistance of Candida glabrata has been reported during fluconazole therapy.
Resistance to Fluconazole has been reported to occur more frequently than resistance to ketoconazole or Itraconazole, which may be related to the drug’s widespread use. Cross-resistance with other azoles and with amphotericin B has also been reported.
Fluconazole resistance has also been reported in Cryptococcus neoformans and Histoplasma capsulation. Histoplasmosis developed during treatment with Fluconazole in a patient with HIV infection. Fluconazole-resistant C. neoformans was isolated from an immunocompetent patient not previously exposed to azole antifungals.
Fluconazole Drug-Drug Interactions
Alfentanil
In a randomized, double-blind, placebo-controlled, crossover study in nine subjects, Fluconazole 400 mg reduced the clearance of alfentanil 20 micrograms/kg by 55% and increased alfentanil-induced subjective effects.
Amitriptyline
An interaction of Fluconazole with amitriptyline has been reported.
- A 12-year-old boy with prostatic rhabdomyosarcoma had episodes of syncope periodically over 7 months while taking Fluconazole for chemotherapy-induced mucositis. He had taken Fluconazole in the past without problems. Still, he had also taken a stable dose of amitriptyline for neuropathic pain. On withdrawal of amitriptyline, he had no further episodes. The effect was confirmed by readministration.
Concurrent administration of Fluconazole probably increases exposure to amitriptyline. Three reports of adults have shown increased amitriptyline plasma concentrations with concurrent fluconazole administration; in one patient, a 57-year-old woman, the QT interval was prolonged, and torsade de pointes occurred.
Amphotericin
In vitro studies and experiments in animals have given conflicting results relating to potential antagonism between the effects of Fluconazole and amphotericin on Candida species. However, large, randomized, double-blind comparisons of Fluconazole with and without amphotericin for 5 days in non-neutropenic patients with candidemia showed no evidence of antagonism but faster clearance of the organism from the blood and a trend toward an improved outcome in those who received the combination.
Antacids
Fluconazole absorption after oral administration is not influenced by gastric pH; thus, antacids such as co-magaldrox do not affect it.
Antihistamines
The concurrent use of terfenadine with Fluconazole can lead to dangerously high terfenadine concentrations, resulting in cardiotoxicity. It is suspected that the same may happen with astemizole.
Benzodiazepines
Twelve healthy men were studied in a randomized, double-blind, four-way crossover study to determine the interaction of Fluconazole with bromazepam. They received single oral or rectal doses of bromazepam (3 mg) after a four-day pretreatment with oral Fluconazole (100 mg/day) or placebo. Fluconazole caused no significant changes in the pharmacokinetics and pharmacodynamics of oral or rectal bromazepam.
Fluconazole increased blood concentrations of midazo-lam and triazolam.
The effects of Fluconazole (400 mg loading dose followed by 200 mg/day) on the kinetics of midazolam have been studied in 10 mechanically ventilated adults receiving a stable infusion of midazolam. Concentrations of midazolam were increased up to four-fold after the start of fluconazole therapy; these changes were most marked in patients with renal insufficiency. During the study, the ratio of a-hydroxy midazolam to midazolam progressively fell. The authors concluded that in ICU patients receiving Fluconazole, reduction of the dose of midazolam should be considered if the degree of sedation increases.
In a study of the pharmacokinetics and pharmacodynamics of oral midazolam 7.5-15 mg, switching from inhibition of metabolism by Itraconazole 200 mg/day to induction of metabolism by rifampicin 600 mg/day caused an up to 400-fold change in the AUC of oral midazolam.
Calcium Сhannel Blockers
Fluconazole enhanced the blood pressure-lowering effects of nifedipine by increasing its plasma concentrations in a 16-year-old patient with malignant pheochromocytoma taking chronic nifedipine for arterial hypertension who was given Fluconazole for Candida septicemia.
Carbamazepine
Fluconazole can cause carbamazepine toxicity, presumably by inhibition of CYP3A4.
- A 33-year-old man on stable therapy with carbamazepine (400 mg tds) for a seizure disorder became stuporose due to carbamazepine toxicity after taking Fluconazole 150 mg/day for 3 days. Withdrawal of both drugs resulted in a fall in carbamazepine concentrations (maximum concentration 25 µg/ml) and a return of the patient’s baseline mental status. Carbamazepine was restarted, and the patient had no further adverse events.
- Carbamazepine serum concentrations increased during concomitant fluconazole administration (400 mg/day) in a 38-year-old man.
Ciclosporin
Fluconazole can increase ciclosporin concentrations by inhibiting CYP3A4. In some studies, minimal or no effects were recorded, but Fluconazole increased ciclosporin concentrations in others. Differences in the dosage and duration of fluconazole treatment could have explained these discrepancies. For example, there was no interaction at a fluconazole dosage of 100 mg/day, but high dosages of Fluconazole (400 mg/day or more) increased blood ciclosporin and tacrolimus concentrations.
The interaction of ciclosporin with Fluconazole has been retrospectively evaluated in 19 kidney and pancreas/kidney transplant recipients. Both intravenous and oral Fluconazole altered the blood concentration of ciclosporin. Five subjects did not have a significant interaction, and 15 did. No patient had nephrotoxicity or transplant rejection related to antifungal therapy.
The effects of higher dosages of Fluconazole on ciclosporin immunosuppression have been investigated in six renal transplant patients in a prospective, unblinded, crossover study. Baseline renal function, ciclosporin AUC, Cmax, Cmm, fmax, and clearance were compared with those 2,4 and 7 days after starting Fluconazole orally in a dosage of 200 mg/ day. From day 8 onwards, the ciclosporin dose was reduced by 50%, and the above parameters were repeated on day 14. The results are shown in Table 1. On repeated-measures ANOVA, only the AUC and Cmax on day 4 of Fluconazole were significantly higher than on day 0. There were no significant changes in ciclosporin clearance and fmax. The authors concluded that changes in Cmin may not be sensitive enough to detect the described interaction and suggested monitoring the AUC near day 4 of treatment to guide ciclosporin dosage adjustments in all patients taking concomitant Fluconazole.
Cimetidine
Fluconazole absorption after oral administration is not influenced by gastric pH; thus, cimetidine has no effect.
Clarithromycin
The effects of Fluconazole and clarithromycin on the pharmacokinetics of rifabutin and 25-O-desacetylrifabutin have been studied in 10 HIV-infected patients who were given rifabutin 300 mg qds in addition to Fluconazole 200 mg qds and clarithromycin 500 mg qds. There was a 76% increase in the plasma AUC of rifabutin when either Fluconazole or clarithromycin was given alone and a 152% increase when both drugs were given together. The authors concluded that patients should be monitored for adverse effects of rifabutin when it is co-administered with Fluconazole or clarithromycin.
Cyclophosphamide
Cyclophosphamide is a prodrug that is metabolized by CYP450 enzymes to produce alkylating species, which are cytotoxic, and the extent of cyclophosphamide metabolism correlates with both treatment efficacy and toxicity. In vitro studies in six human liver microsomes showed that the IC50 of Fluconazole for reduction of 4-hydroxycyclophosphamide production was 9-80 µmol/l.
A retrospective study in 22 children with cancers addressed the potential interaction between Fluconazole and cyclophosphamide. Children with an established profile of cyclophosphamide metabolism who were not receiving other drugs known to affect drug metabolism were selected; 9 were taking Fluconazole, and 13 were controls. The plasma clearance was significantly lower in patients taking concomitant Fluconazole (2.4 versus 4.2 1/ hour/m. It is unclear whether this interaction is associated with reducing the therapeutic efficacy of cyclophosphamide.
Doxorubicin
The effect of Fluconazole on the plasma pharmacokinetics of doxorubicin has been investigated in a randomized crossover study in non-human primates. Fluconazole (10 mg/kg/day) was given intravenously for 4 days before doxorubicin (2.0 mg/kg intravenously). Pretreatment with Fluconazole did not affect the pharmacokinetics of doxorubicin, and the incidence of severe neutropenia (absolute neutrophil count below 0.5 x 109/1) was higher with doxorubicin alone than with the combination of doxorubicin and Fluconazole. Thus, Fluconazole does not appear to contribute to the marrow-suppressive effects of doxorubicin.
Flucytosine
The concurrent use of Fluconazole and flucytosine may have an additive effect. This combination could help treat cryptococcal meningitis.
HIV Protease Inhibitors
The pharmacokinetic interaction of Fluconazole 400 mg OD and indinavir 1000 mg TDs was evaluated in an 8-day placebo-controlled crossover study; there was no significant interaction.
The effect of Fluconazole on the steady-state pharmacokinetics of ritonavir and saquinavir has been studied in patients infected with HIV-1. They received the protease inhibitor (saquinavir 1200 mg tds, n = 5, or ritonavir 600 mg bd, alone on day 1 and then with Fluconazole 400 mg on day 2 and 200 mg on days 3-8. The median increase in saquinavir AUC was 50%, and the median increase in Cmax was 56%. In contrast, Fluconazole did not affect the disposition of ritonavir.
Methadone
An interaction between Fluconazole and methadone (a substrate of CYP3A4, CYP2C9, and CYP2C has been reported.
While taking a stable dose of methadone, a 60-year-old man with advanced cancer developed respiratory depression 2 days after receiving intravenous Fluconazole for refractory oral candidiasis. Intravenous naloxone reversed the respiratory depression.
In a randomized, double-blind, placebo-controlled study in 25 patients, Fluconazole 200 mg/day increased methadone concentrations, but patients treated with Fluconazole did not have signs or symptoms of significant narcotic overdose.
Omeprazole
Fluconazole absorption after oral administration is not influenced by gastric pH; thus, omeprazole has no effect. Omeprazole is extensively metabolized in the liver by 5-hydroxylation and sulfoxidation reactions, catalyzed predominantly by CYP2C19 and CYP3A4, respectively. Fluconazole is a potent competitive inhibitor of CYP2C19 and a weak inhibitor of CYP3A4. The effect of Fluconazole on the pharmacokinetics of a single oral dose of omeprazole 20 mg has been evaluated after a single oral dose of Fluconazole 100 mg and after 4 days of oral administration of 100 mg/day in 18 healthy male volunteers. Fluconazole increased the Cmax and the mean AUC of omeprazole and prolonged its half-life (2.59 versus 0.85 hours).
Oral Contraceptives
Fluconazole did not significantly alter the pharmacokinetics of ethinylestradiol or norgestrel, and the investigators interpreted this finding as suggesting that treatment with Fluconazole in a user of oral contraceptives would not increase the risk of pregnancy. However, the study used a 50 mg dose of Fluconazole, and experience with higher dosages has shown different results.
In another study, Fluconazole reduced the systemic availability of ethinylestradiol in an oral contraceptive, but the doses used were not reported.
In an open, crossover study of 10 young, healthy subjects, Fluconazole 150 mg increased the serum concentrations of ethinylestradiol 30-35 µg/day.
The potential pharmacokinetic interaction between Fluconazole 300 mg once weekly and an oral contraceptive containing ethinylestradiol and norethindrone (Ortho-Novum 7/7/7; Ortho-McNeil Pharmaceutical Inc, Raritan, NJ) has been studied in a placebo-controlled, double-blind, randomized, two-way, crossover study in 26 healthy women aged 18-36 years. During the first cycle, they took the oral contraceptive only. During the second cycle, they were assigned randomly to oral contraceptive + Fluconazole or oral contraceptive + placebo. In the third cycle, they were given another treatment.
Fluconazole caused small but statistically significant increases in the AUCo-24 of ethinylestradiol and norethindrone. Those given Fluconazole experienced no adverse events related to treatment.
It, therefore, appears that there is no threat of contraceptive failure because of concomitant fluconazole administration.
Phenytoin
Co-administration of Fluconazole and phenytoin resulted in markedly higher phenytoin concentrations.
Rifamycins
The combination of rifampicin with Fluconazole has insignificant effects.
Statins
The effects of Fluconazole on plasma fluvastatin and pravastatin concentrations have been studied in two separate, randomized, double-blind, two-phase, crossover studies. Healthy volunteers were given oral Fluconazole (400 mg on day 1 and 200 mg on days or placebo. On day 4, they took a single oral dose of fluvastatin 40 mg or pravastatin 40 mg.
Fluconazole increased the plasma AUC and the half-life of fluvastatin by 80% but had no significant effects on the pharmacokinetics of Pravastatin. The mechanism of the prolonged elimination of fluvastatin was probably inhibition of CYP2C9. In contrast, Pravastatin appears not susceptible to interactions with Fluconazole and other CYP2C9 inhibitors.
Fluconazole’s effect on rosuvastatin’s pharmacokinetics has been investigated in a randomized, double-blind, two-way, crossover, placebo-controlled study. Healthy male volunteers were given 200 mg/day fluconazole or matching placebo for 11 days; rosuvastatin 80 mg was co-administered on day 8. Plasma concentrations of rosuvastatin, N-desmethyl rosuvastatin, and active and total HMG-CoA reductase inhibitors were measured up to 96 hours after the dose.
Fluconazole increased the AUC and Cmax of rosuvastatin by 14% and 9% respectively. Limited data available for the N-demethylated metabolite showed that the Cmax fell by about 25%. Fluconazole did not affect the proportion of circulating active or total HMG-CoA reductase inhibitors accounted for by circulating rosuvastatin. Thus, Fluconazole produced only minor changes in rosuvastatin kinetics, which were not clinically relevant.
Sulfonylureas
The concurrent administration of Fluconazole with tolbutamide resulted in increased tolbutamide concentrations.
- A 56-year-old HIV-positive patient with diabetes mellitus taking gliclazide 160 mg/day developed severe hypoglycemia when treated with co-trimoxazole 480 mg/day and Fluconazole 200 mg/day. The authors speculated that Fluconazole might have inhibited gliclazide metabolism by inhibiting CYP2C9.
The effects of Fluconazole and fluvoxamine on the pharmacokinetics and pharmacodynamics of glimepiride have been studied in a randomized, double-blind, crossover study in 12 healthy volunteers who took Fluconazole 200 mg/day (400 mg per day, fluvoxamine 100 mg/day, or placebo once daily for 4 days. On day 4, they took a single oral dose of glimepiride 0.5 mg.
Fluconazole increased the mean total AUC of glimepiride to 238%, the peak plasma concentration to 151% of control values, and the half-life of glimepiride was prolonged from 2.0 to 3.3 hours. This was probably due to inhibition of CYP2C9-mediated biotransformation of glimepiride by Fluconazole. However, Fluconazole did not cause statistically significant changes in the effects of glimepiride on blood glucose concentrations.
Tacrolimus
Since tacrolimus is metabolized by intestinal and hepatic CYP3A4, drugs that inhibit CYP3A4 can reduce the metabolism of tacrolimus and increase tacrolimus blood concentrations.
The effect of Fluconazole on the blood concentrations of tacrolimus has been investigated in eight liver transplant patients in whom prophylactic Fluconazole (200 mg/day) was withdrawn because of rises in hepatic transaminases, renal dysfunction, or eosinophilia (n = 1 each). Calculated tacrolimus concentrations fell by 13-81% (median 41%) between the fourth and ninth days after withdrawal of Fluconazole. Tacrolimus blood concentrations should be carefully monitored, and dosages should be increased as necessary after the withdrawal of Fluconazole.
The interaction of tacrolimus with Fluconazole has been retrospectively evaluated in 19 kidney and pancreas/kidney transplant recipients. Both intravenous and oral Fluconazole altered the blood concentration of tacrolimus. Five subjects did not have a significant interaction, and 15 did. No patient had nephrotoxicity or transplant rejection related to antifungal therapy.
- A 17-year-old man with cystic fibrosis who took Itraconazole after a lung-liver transplant had high trough concentrations of tacrolimus despite the relatively low dosage (0.1-0.3 mg/kg/day).
- A patient taking tacrolimus 0.085 mg/kg bd with itraconazole 200-400 mg/day developed ketoacidosis, neutropenia, and thrombocytopenia, requiring the withdrawal of both drugs.
- A 34-year-old renal transplant recipient taking a stable regimen of tacrolimus and methylprednisolone was given itraconazole 100 mg bd for a urinary tract yeast infection. Concomitant therapy with Itraconazole led to a marked increase in tacrolimus trough concentrations on the second day of therapy (from 13 to 21 µg/ml) and an increase in serum creatinine concentrations, necessitating a 50% dosage reduction of tacrolimus.
When Itraconazole was withdrawn, its effect on the kinetics of tacrolimus took 12 days to reverse. The inhibitory effect of Itraconazole occurred quickly, while the time of disappearance was much longer, which is important for clinical management. Thus, during the co-administration of Itraconazole with tacrolimus, close monitoring of tacrolimus blood concentrations and careful dosage adjustments are essential to avoid toxicity.
Warfarin
Co-administration of Fluconazole and warfarin has led, in some cases, to the prolongation of the prothrombin time.
Zidovudine
The combination of Fluconazole with valproic acid inhibited zidovudine glucuronidation in human hepatic microsomes in vitro more than other drugs, such as atovaquone and methadone.
Adverse Effects
Read indications for use if you want to order Fluconazole online.
Adverse effects reported with Fluconazole most commonly affect the gastrointestinal tract, including abdominal pain, diarrhea, flatulence, nausea and vomiting, and taste disturbance. Other adverse effects include headache, dizziness, leucopenia, thrombocytopenia, hyperlipidaemias, and raised liver enzyme values. Serious hepatotoxicity has been reported in patients with severe underlying diseases such as AIDS or malignancy. Anaphylaxis and angioedema have been reported rarely. Skin reactions are rare, but exfoliative cutaneous reactions such as toxic epidermal necrolysis and Stevens-Johnson syndrome have occurred, more commonly in patients with AIDS.
Alopecia
Alopecia has occasionally been reported in patients receiving Fluconazole, especially during prolonged use.
Effect on Electrolyte Balance
Hypokalaemia was associated with Fluconazole in 3 patients with acute myeloid leukemia.
Effects on the Heart
Prolonged QT interval and torsade de pointes have been reported rarely in patients receiving Fluconazole.
Effects on the Liver
Although severe hepatic reactions to Fluconazole are rare, they have been reported, especially in patients with severe underlying diseases or hepatic dysfunction. Elevated liver enzymes are commonly found, and there have been reports of jaundice. Hepatic necrosis has been seen rarely post-mortem in patients with severe underlying disease who had received Fluconazole. In one such patient, hepatotoxicity was concluded to be dose-dependent.

Hypersensitivity
Desensitization has been successfully carried out in an AIDS patient who exhibited hypersensitivity to both Fluconazole and Itraconazole. Over seven days, gradually increasing oral doses of Fluconazole (starting at 5 mg daily) were given; thereafter, the dosage was maintained at 400 mg daily. No adverse reactions were noted during the desensitization period or in the three months before the report’s publication.
Precautions
Before buying Fluconazole online, read information about the drug.
Fluconazole should be used with caution in patients with impaired hepatic or renal function. Abnormalities in hematological, hepatic, and renal function tests have been observed in patients with serious underlying diseases such as AIDS or malignancy. Cases of torsade de pointes and QT prolongation have been reported rarely, and caution is advised when giving Fluconazole to patients with proarrhythmic conditions. Teratogenicity has occurred in animals given high doses of Fluconazole, and its use is not recommended during pregnancy.
Breastfeeding
Fluconazole is distributed into breast milk, achieving concentrations similar to those found in maternal plasma. However, licensed product information does not recommend its use in breastfeeding women. In one report, no untoward effects, other than a slight increase in lactase dehydrogenase level, were seen in an infant exposed to Fluconazole in breast milk for six weeks. The American Academy of Pediatrics considers fluconazole tablets to be usually compatible with breastfeeding.
Pregnancy
High (toxic) doses of Fluconazole, Itraconazole, and ketoconazole have been reported to be teratogenic in rodents.
Although there is little information about the use of these drugs in human pregnancy, there is a report of a woman who took Fluconazole 400 mg daily throughout pregnancy and who gave birth to an infant with severe craniofacial and limb abnormalities. The abnormalities resembled those associated with the Antley-Bixler syndrome, a genetic disorder. Still, a teratogenic effect could not be excluded.
Although prescription-event-monitoring studies of Fluconazole did not reveal adverse effects on the fetus, congenital abnormalities have occurred in infants whose mothers were given high doses of Fluconazole for 3 months or more. Data collected by the manufacturer, relating to 198 women exposed to Itraconazole during the first trimester of pregnancy, indicated that the malformation rate for both exposed women and matched controls was within the expected baseline risk for the general population.
Nevertheless, the manufacturers recommend avoiding Fluconazole, Itraconazole, and ketoconazole during pregnancy. Licensed product information states that doses of voriconazole equivalent to those used therapeutically are teratogenic and embryotoxic in rodents. Therefore, the manufacturer recommends avoiding voriconazole during pregnancy and that women of child-bearing potential use effective contraception during treatment.
Similar recommendations have been made for posaconazole. Other azole antifungals, including butoconazole, clotrimazole, econazole, miconazole, sulconazole, terconazole, and tioconazole, are reported to be embryotoxic but not teratogenic in rodents given high doses. Many of these drugs are used topically or intravaginally, and the systemic absorption from these routes of administration varies. While these drugs may not necessarily be contraindicated in pregnancy, consideration should be given to these potential risks when choosing antifungal therapy for such patients.
Other Brand Names of Fluconazole
Under what local brands and in what dosages is generic Fluconazole sold in pharmacies in Britain, the United States, and Canada?
In pharmacies in the United States, Great Britain, and Canada, the pharmacists offer you to buy Fluconazole according to your prescription or without a prescription under such brand names and in such strengths and dosage forms:
| UK | US | Canada |
| Azocan 50mg Capsules Azocan 100mg Capsules Azocan 150mg Capsules Azocan-P 150mg Capsules Boots Thrush 150mg Capsule Diflucan 150 Capsules Fluconazole 50mg Capsules Fluconazole 100mg Capsules Fluconazole 150mg Capsules Fluconazole 200mg Capsules | Diflucan 50mg Tablets Diflucan 100mg Tablets Diflucan 150 mg Tablets Diflucan 200 mg Tablets Fluconazole 50mg Tablets Fluconazole 100mg Tablets Fluconazole 150 mg Tablets Fluconazole 200 mg Tablets Fluconazole for Oral Suspension 50mg/5ml Fluconazole Oral Suspension 200mg/5ml | Diflucan Tab 50mg Diflucan Tab 100mg Diflucan Inj 2 mg/ml Diflucan PWS 50mg/5ml Fluconazole Injection 2mg/ml Mylan-Fluconazole 50mg Tablets Mylan-Fluconazole 100mg Tabs Novo-Fluconazole 50mg Tablets Novo-Fluconazole 100mg Tabs Novo-Fluconazole 150mg Tabs |
Proprietary Preparations
| Country | Medication Names |
|---|---|
| Argentina | Candimicol; Damicol; Femixol; Fluconovag; Fluzol; Fungocina; Fungototal; Honguil Plus; Klonazol; Micolis Novo; Mutum; Naxo C; Nifurtox; Niofen; Periplum; Ponaris; Proseda F; Triflucan |
| Australia | Diflucan; Dizole; Fluzole; Ozole |
| Austria | Diflucan; Diflucohexal; Difluzol; Fluconabene; Flucosept; Flucozal; Fungata |
| Belgium | Diflucan; Fungimed |
| Brazil | Candix; Candizol; Celozol; Farmazol; Floltec; Flucanil; Flucanol; Flucazol; Flucodan; Flucoltrix; Flucomed; Fluconal; Fluconax; Fluconeo; Flucozen; Flucozix; Flunal; Flunazol; Flutec; Fungnon; Glyflucan; Helmicin; Monipax; Pantec; Pronazol; Riconazol; Triazol; Unizol; Zelix; Zolanix; Zolmic; Zolstatin; Zoltec; Zoltren |
| Canada | Diflucan |
| Chile | Diflucan; Felsol; Flucoxan; Fluctin; Fungimax; Ibarin; Micofin; Microvaccin; Plusgin; Tavor |
| Czech Republic | Diflazon; Diflucan; Fluco; Forcan; Mycomax; Mycosyst; Mykohexal |
| Denmark | Conasol; Diflucan; Fungal |
| Finland | Diflucan |
| France | Beagyne; Triflucan |
| Germany | Canex; Diflucan; Fluc; Flucobeta; Flucoderm; FlucoLich; Flunazul; Fungata |
| Greece | Azoflu; Farviron; Figalol; Flucocaps; Flucodrug; Fluconapen; Flusenil; Funadel; Fungo; Fungustatin; Fungusteril; Fuxilidin; Gynosant; Hadlinol; Medoflucon; Mycazole; Rifagen; Stabilanol; Tierlite; Varmec; Zidonil |
| Hong Kong | Diflucan; Flucoric; Flucozal; Fludicon; Forcan; Lucon; Nofung; Stalene |
| Hungary | Dermyc; Diflazon; Diflucan; Flucohexal; Flucoric; Mycosyst; Nofung |
| India | Flumyc; Fluzon; Forcan; Logican; Nipcan; Syscan |
| Indonesia | Cancid; Candizol; Cryptal; Diflucan; Flucess; Flucoral; Govazol; Zemyc |
| Ireland | Diflazole; Diflucan; Flucandid; Flucol |
| Israel | Diflucan; Flucanol; Trican; Triflucan |
| Italy | Biozolene; Diflucan; Elazor |
| Japan | Diflucan |
| Malaysia | Biozole; Diflucan; Flucoric; Flugal; Fukole; Medoflucon; Stalene; Zolstan |
| Mexico | Afungil; Bioxel; Candizol; Diflucan; Difusel; Fectrin; Flucoxan; Fludisol; Fluhexal; Flukezol; Fluxes; Fluxicap; Fluzor; Funser; Lanfluzol; Neofomiral; Ongicil; Oxifungol; Solarisol; Terplex; Zoldicam |
| The Netherlands | Diflucan |
| Norway | Diflucan |
| New Zealand | Canesten Fluconazole; Diflucan; Flucazole |
| Philippines | Diflucan; Funzela; Syscan |
| Poland | Diflucan; Flucofast; Flumycon; Mycomax; Mycosyst |
| Portugal | Azoflune; Diflucan; Fludocel; Maxflin; Reforce; Supremase |
| Russia | Diflazon (Дифлазон); Diflucan (Дифлюкан); Flucostat (Флюкостат); Flucozan (Флукозан); Flumicon (Флюмикон); Fungolon (Фунголон); Funzole (Фунзол); Medoflucon (Медофлюкон); Mycoflucan (Микофлюкан); Mycomax (Микомакс); Mycosyst (Микосист); Syscan (Цискан) |
| Singapore | Diflucan; Medoflucon; Mycorest; Omastin; Stalene |
| Spain | Diflucan; Lavisa; Loitin; Solacap |
| Sweden | Diflucan |
| Switzerland | Diflucan; Flucazol; Fluconax; Flunizol; Mykantol |
| Thailand | Biozole; Diflucan; Flucozole; Fludizol; Flunco; Funa; Kyrin; Stalene |
| Turkey | Biocanol; Candidin; Flucan; Fluzole; Fungan; Kandizol; Lumen; Triflucan; Trizol; Zolax |
| United Kingdom | Canesten Oral; Diflucan |
| United States | Diflucan |
| Venezuela | Aflumicort; Albesin; Diflucan; Flucess; Flucon; Flunal; Fluval; Fugin; Fungomax; Funizol; Micoflux; Mutum; Zolstan |
Multi-ingredient Preparations
| Country | Medication Names |
|---|---|
| Argentina | Gynerium UD |
| Australia | Canesoral Duo |
| India | Forcan TZ; Orflaz Kit; Safkit |
| Mexico | Afumix |



 (91 votes, average: 3.76 out of 5)
(91 votes, average: 3.76 out of 5)















