What does the infectious disease specialist mean by parasitic infection?
Most infectious agents fulfill the definition of a parasite: an organism that grows, feeds, and shelters on or in a different organism and contributes nothing to the host. However, medical science has created the classification “parasite” to include a complex group of nonfungal eukaryotic human pathogens.
Unlike fungi, parasites have no cell wall and are often motile. In addition, many parasites require two or more host species to complete their life cycle, and they reproduce both sexually and asexually.
The host in which sexual reproduction takes place is called the “definitive host,” and the one in which asexual reproduction occurs is called the “intermediate host.” Previously, parasitic infections were almost exclusively a health problem in developing countries with poor sanitation.
However, with the current marked rise in international travel and increased military deployments to endemic areas, these infections are now increasingly being diagnosed in the United States, Europe, and other developed countries. The incidence of symptomatic parasitic infections has also increased because of the ever-increasing population of immunocompromised hosts.
Organ transplant, cancer chemotherapy, and infection with HIV all lead to depressed cell-mediated and humoral immunity, allowing dormant parasites to reactivate and cause disease. More than ever before, thorough travel and exposure histories are critical steps in accurately diagnosing parasitic infections.
An awareness of geography and environmental conditions and a familiarity with the life cycles of various parasites are all required for proper diagnosis and treatment.
Blood Protozoa
Potential Severity
Hours can make the difference between life and death. Rapid diagnosis and treatment are critical.
Malaria
Prevalence
The combination of deteriorating political and economic conditions in the countries of sub-Saharan Africa and the development of chloroquine drug resistance in many parts of the world have resulted in a resurgence of malaria. Climate change and the increased resistance of mosquitoes to insecticides have also contributed to this trend.
The worldwide annual incidence of malaria is between 300 and 500 million cases, causing between 1 and 2 million deaths. Areas with significant numbers of malaria cases include Africa, the Middle East, India, Southeast Asia, South America, Central America, and parts of the Caribbean.
Chloroquine resistance is now the rule in most countries. Plasmodium falciparum in Southeast Asia is frequently resistant not only to chloroquine, but also to pyrimethamine-sulfadoxine, mefloquine, and halo-fantrine. Areas in which P. falciparum remains sensitive to chloroquine include Central America and the Caribbean, in particular Haiti. In the United States, secondary cases have been reported around airports, and an outbreak of P. vivax was recently described in Palm Beach, Florida.
Because the sensitivity patterns of malaria continue to change annually, the Centers for Disease Control and Prevention (CDC) should be consulted for the most up-to-date information (Web address: www.cdc.gov/travel).
Malaria Epidemiology and Life Cycle
Humans contract malaria after being bitten by the anophiline female mosquito. Only the female mosquito takes a blood meal, because blood is required for the development of the mosquito egg. Certain strains appear to be more efficient transmitters of disease.
In particular Anophelesgambiae and A funestus are thought to account for the high transmission rates in sub-Saharan Africa. These strains are not present in South America and Southeast Asia where transmission rates are lower. Clearly the larger the number of mosquito bites a person receives, the greater the risk of contracting malaria.
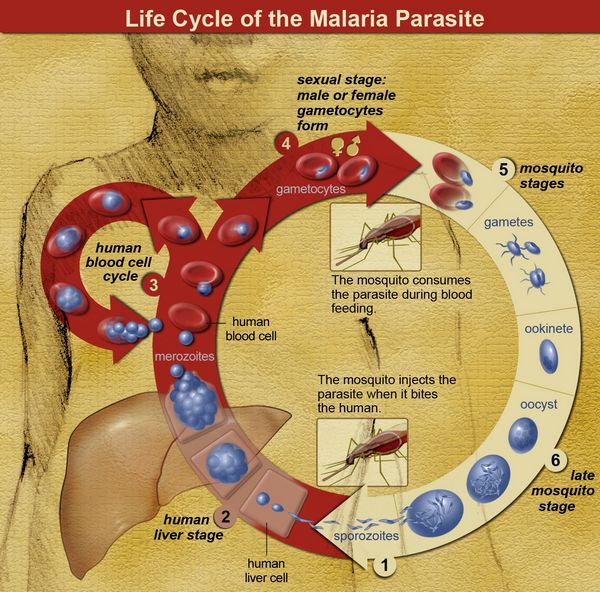
Therefore, in addition to chemoprophylaxis (discussed later in this subsection), mosquito netting, long-sleeved shirts, long pants, insect repellant, and staying in a protected environment during the times of the day when mosquitoes are at their most active are all recommended as preventive measures. The sporozoites introduced into the human bloodstream by the female anophiline mosquito quickly travel to the liver and invade hepatocytes.
Sporozoites contain a specific protein thought to be critical for binding and entry into hepatocytes. This circumsporozoite protein binds to specific host-cell membrane receptors (heparin sulfate proteoglycans and low-density lipoprotein receptor-related protein). Within the hepatocytes, most sporozoites mature to tissue schizonts. Some sporozoites become dormant, This dormant form, called a hypnozoite takes 6 to 11 months to activate into a tissue schizont.
Each schizont-infected hepatocyte then produces 10,000 to 30,000 merozoites that are released into the bloodstream following cell lysis. Each merozoite can invade a single red blood cell and asexually replicate five times over 48 to 72 hours to produce 32 merozoites. The red blood cell then undergoes lysis, releasing the newly formed merozoites, which can infect additional red blood cells. Under ideal conditions, a single sporozoite could theoretically account for the infection of nearly 1 million red blood cells (many of the free merozoites are intercepted by host macrophages, thus reducing the efficiency of red cell infection).
As observed with sporozoite entry into hepatocytes, a specific protein on the merozoite surface (erythro-cyte-binding antigen 175 in P. falciparum and Pvl35 in P. vivax) binds to a specific red blood cell membrane receptor (glycophorin A in P. falciparum and Duffy factor in P. vivax) allowing attachment and entry. Once the merozoite enters the red blood cell, it matures to a tropho-zoite. This form looks like a signet ring and can readily be seen in parasitized red blood cells following Giemsa or Wright staining. As the trophozoite matures, it loses its signet ring morphology, becoming larger and subsequently developing into a red blood cell schizont, which then splits into multiple merozoites. Upon entry into the red blood cell, some merozoites mature into sexual forms called gametocytes rather than into asexual forms.
The male form is smaller and is called a microgametocyte; the larger female form is called a macrogametocyte. Because sexual mating does not occur in the human host, but only in the mosquito, the mosquito is considered the definitive host, and humans are considered the intermediate host. Once fertilization occurs, a zygote is formed that subsequently develops into an oocyst. The oocyst then forms thousands of infectious sporozoites that gain entry into the mosquito salivary gland, where they are transmitted to the human host.
Life cycle Differences Between the Various Plasmodium Species
P. falciparum is the most common, and most dangerous, form of malaria. Unlike the sporozoites of other strains, all falciparum sporozoites that enter the liver remain active and develop into tissue schizonts that proceed to form thousands of merozoites.
And unlike the merozoites of other strains, P. falciparum merozoites can infect red blood cells of all ages, explaining the high level of parasitized red blood cells observed in falciparum malaria. Moreover, in the non-falciparum forms of malaria only a single merozoite gains entry into a given red cell; in falciparum malaria, multiple merozoites can infect and mature within a single red blood cell.
Once a merozoite has invaded a red blood cell, it rapidly matures, asexually divides, and within 48 hours, lyses the host cell. This rapid asexual reproduction produces a rapid rise in the percentage of infected host red blood cells, and as the percentage of parasitized red blood cells increases, the risk of death or serious complications also increases. P. falciparum is more harmful to the host because invasion by this strain is uniquely associated with the formation of red blood cell membrane knobs that tightly adhere to the vascular endothelium. These knobs express erythrocyte membrane protein 1 on their surface, and this protein binds complement receptor 1 (CR-1) on uninfected red cells, causing red cell clump- AU: ing (“resetting”). These adherent red blood cells block blood flow in small blood vessels, causing severe hypoxic damage, particularly to the brain and kidneys.
Because red blood cell adherence develops as the merozoite matures beyond the early trophozoite stage, other maturation stages of the parasite (with the exception of the banana-shaped gametocytes) are rarely seen in the peripheral blood. P. vivax is the next most common form of malaria. P. malariae is less common, and P. ovale is a rare human infection. When a female anophiline mosquito bites an infected human, gametocytes are taken in with the blood. P. vivax and P. ovalecan form hypnozoites that can remain dormant within the liver for months before becoming active tissue schizonts.
This behavior explains the ability of these strains to relapse 6 to 11 months after initial treatment. P. malariaehas no dormant liver phase, but can persist as a low-level infection for up to 30 years. P. vivax and P. ovale merozoites bind only young red blood cells, having the highest affinity for reticulocytes. P. malariae tends to infect older red blood cells. The inability of these strains to infect a broad age range of red blood cells explains their low level of parasitemia. Furthermore, these three strains do not form knobs and do not obstruct the microcircula-tion, explaining their milder clinical manifestations. In Malaysia, P. knowlesi, a form of malaria formerly thought to infect only monkeys, has been identified in humans. Its trophozoite stage is similar in morphology to that of P. falciparum, and its schizont stage is similar to that of P. malariae.
The level of parasitism can be high, resulting in serious infections. P. knowlesi should be considered in individuals traveling to forested tropical regions where monkeys are known to be infected.
About the Lifecycle of Plasmodium falciparum
P. falciparum is the most dangerous form of malaria because it
- infects red blood cells of all ages and causes high levels of parasitemia.
- induces the formation of knobs on the RBC surface that adhere to vessel walls and to uninfected red blood cells, causing obstruction and local hypoxia.
- can cause severe hemolysis, renal failure, central nervous system damage,and pulmonary edema.
Table . Differences in Malaria Strains
| Strain | Characteristics |
| Plasmodium falciparum | No dormant phase in the liver Multiple”signet ring”trophozoites per cell High percentage (>5%) of parasitized red blood cells Development stages other than the early signet-ring trophozoite and mature gametocyte not seen |
| Plasmodium vivax and Plasmodium ovale | Dormant liver phase Single signet-ring trophozoites per cell Schuffner’s dots in the cytoplasm Low percentage (>5%) of parasitized red blood cells All developmental stages seen Red blood cells often appear enlarged in the later stages |
| Plasmodium malariae | No dormant phase Single signet-ring trophozoite per cell Very low level parasitemia All developmental stages seen Red blood cells normal size |
Genetic and Other Determinants of Susceptibility to Malaria
In areas in which malaria is endemic, the high prevalence of genetic traits that reduce susceptibility to malaria serves as remarkable examples of Darwinian evolution. Specific mutations that affect the surface proteins, cytoskeleton, and hemoglobin of red blood cells all interfere with Plasmodium invasion, survival, and spread, and thereby provide a survival advantage to the infected host. Absence of the Duffy blood group antigen blocks invasion by P. vivax. This strain of malaria must bind to this particular blood group antigen to gain entry into red blood cells. A significant number of black Africans are Duffy-negative and are resistant to P. vivax.
Individuals with mutations in CR-1 demonstrate reduced rosetting in association with P. falciparum and have a decreased propensity to produce cerebral malaria. Individuals with hereditary ovalocytosis, elliptocytosis, and spherocytosis all have defects in specific red blood cell cytoskeleton proteins, and these defects interfere with entry and release of the malaria parasite. A broad range of hemoglobinopathies are protective against malaria.
The high prevalence of sickle cell disease and sickle cell trait in Africa illustrates the frighteningly efficient selective powers of the deadly P. falciparum parasite. Parasite growth is slowed in cells with sickle cell hemoglobin (Hb S). In addition, when parasitized red blood cells that contain Hb S form membrane knobs and become trapped in small vessels, oxygen tension decreases, and the Hb S polymerizes, resulting in sickling of red blood cells. The polymerization of Hb S kills the P. falciparum parasite, preventing the infection from progressing.
As a consequence, people with sickle cell trait and sickle cell disease are resistant to severe P. falciparum infection. Because the other strains of malaria do not form knobs and do not become trapped in blood vessels, Hb S does not protect against P. vivax, P. ovale, or P. malariae. A number of other hemoglobinopathies including Hb C, Hb E, a-thalassemia, and to a lesser extent, β-thalassemia reduce the severity of P. falciparum, accounting for their increased prevalence in endemic areas. Neonates are protected from severe malaria as a consequence of fetal hemoglobin, which interferes with the intracellular growth of P. falciparum. In areas that have a high incidence of malaria, the indigenous population is continually exposed the parasite, resulting in a high level of immunity. In these regions, severe disease is rare.
However, because the immune response to malaria is short-lived, immunity wanes in regions in which malaria has been controlled and the attack rate is low. Paradoxically, the percentage of patients developing severe disease increases in these regions. Tourists with no previous exposure to malaria are at highest risk for life-threatening disease. Pregnant women and their fetuses are also at risk. P. falciparum binds to chondroitin sulfate A in the inter-villous space of the placenta, causing hemolytic anemia, which leads to low birth weight infants.
About Genetics and Other Factors that Affect Susceptibility to Malaria
- Surface proteins on red blood cells: a) Individuals negative for the Duffy blood group antigen are resistant to Plasmodium vivax. b) Complement receptor 1 mutations reduce the severity of P. falciparum infection.
- Cytoskeleton defects in red blood cells are protective: a) Hereditary ovalocytosis b) Hereditary elliptocytosis c) Hereditary spherocytosis
- Hemoglobinopathies confer resistance: a) Sickle cell disease and sickle cell trait are resistant to P. falciparum. b) Other hemoglobin mutations and fetal hemoglobin are also resistant to P. falciparum.
- Low-level immunity increases the risk for severe disease: a) Population immunity wanes in areas with low attack rates. b) Tourists lack immunity. c) In pregnant women, the placenta is affected, resulting in low birth weight infants.
Clinical Presentation
CASE 1
A married couple was sailing in the Caribbean near Jamaica with their three children. They lived primarily on their boat, but took several day trips to a small island off the coast of Jamaica. They noted some mosquito bites and ate some fruit while on the island. The man and the woman both suddenly came down with fever, chills, muscle aches, and loss of appetite. About 3 days into the illness, the man became jaundiced and began passing dark urine. The family sought treatment from a local Jamaican physician, who diagnosed hepatitis secondary to ingestion of a toxic food. Two days later, the man became comatose and died.
The woman was referred to the university hospital for possible liver transplant. On further questioning, the medical staff learned that none of the children were sick despite eating the same diet. The family had begun a course of malaria prophylaxis. However, the parents had developed side effects from the chloroquine and had discontinued prophylaxis 2 weeks before the onset of their illness. Thin smears of the woman’s blood revealed many signet-ring trophozoites, with a parasitemia level estimated to be 10%. She was treated with intravenous quinine and rapidly improved. In retrospect, her husband was determined to have died of untreated blackwater fever.
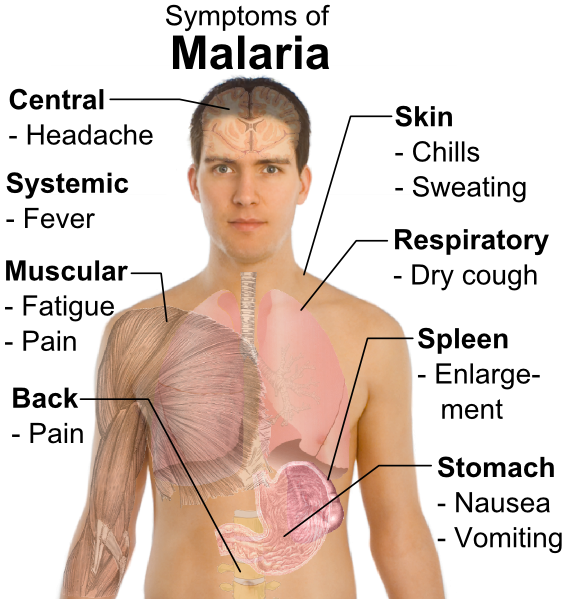
The clinical manifestations of malaria are nonspecific. If the exposure history is not appreciated, the infection can be mistaken for other febrile illnesses. The incubation period is generally 9 to 40 days, but it may be prolonged in cases of non-falci-parum malaria (6 to 12 months in P. vivax, and years for P. malariae and P. ovale). The hallmark of all forms of malaria is fever.
Fever can occur at regular 2 to 3-day intervals in P. vivax and P. malariae, or in a more irregular pattern with P. falciparum. Fever generally occurs soon after lysis of the red blood cells and release of the merozoites. Three classic stages of the febrile paroxysms have been described:
- The initial “cold stage” occurs 15 to 60 minutes before the onset of fever. During this period, the patient feels cold and has shaking chills.
- These symptoms are followed by the “hot stage,” during which body temperature rises to between 39°C and 4l°C. Fever is associated with lassitude, loss of appetite, and vague pains in the bones and joints. In nonendemic areas, these symptoms are most commonly mistaken for influenza. The clinician must always consider malaria in individuals who develop flu-like symptoms after returning from a developing country. Other symptoms associated with the fever include tachycardia, hypotension, cough, headache, back pain, nausea, abdominal pain, vomiting, diarrhea, and altered consciousness.
- Usually within 2 to 6 hours, symptoms progress to the third “sweating” stage, at which time the patient develops marked diaphoresis, followed by resolution of the fever, profound fatigue, and a desire to sleep.
Other symptoms depend on the strain of malaria. In cases of P. vivttx, P. ovale, and P. malariae, there are a few additional symptoms. However, depending on the prior immune status of the host, individuals with P. falciparum can develop a severe fatal illness similar to that described in case 1. Because P. falciparum infects red blood cells of all ages and induces the formation of knobs on the red blood cell surface that adhere to endothelial cells and obstruct small vessels, this parasite can cause severe damage, particularly to the kidneys, brain, and lungs.
Tourists who have no immunity to P. falciparum and people who have undergone splenec-tomy can develop very high levels of parasitemia that result in profound hemolysis. The marked release of hemoglobin can exceed the metabolic capacity of the liver. The resulting rise in unconjugated bilirubin in the bloodstream produces jaundice. Hemoglobin also may be excreted into the urine, causing the urine to become dark. The combination of jaundice and hemoglobinuria has been called blackwater fever. Severe malaria is commonly complicated by renal failure.
Heavy infection with P. falciparum also results in obstruction of the small arteries in the central nervous system, leading to hypoxia. Hypoglycemia may also contribute to Central nervous system dysfunction. Confusion and obtundation can rapidly progress to coma. Grand mal seizures may also develop. Pulmonary edema is a less common complication of P. falciparum infection, being the result of fluid leakage from pulmonary capillaries into the alveoli.
Diagnosis
Microscopic examination of a Giemsa-stained blood smear remains the primary way to identify malaria. In P. falciparum, blood smears are best taken just after the fever peak, when early ring forms are most abundant in peripheral red blood cells. At other times, P. falciparum becomes trapped in the capillaries and may not be found in the peripheral blood. In P. vivax, P. malariae, and P. ovale, various stages of the parasite are present at all times, and therefore diagnostic smears can be taken at any time.
About Clinical Presentation in Plasmodium Infection
Always consider malaria in the traveler from a developing country who 1. presents with an influenza-like syndrome, 2. presents with jaundice, or 3. presents with confusion or obtundation.
About Laboratory Diagnosis of Malaria
- The focus must be on differentiating falciparum malaria from other forms of the disease.
- Blood smear remains the preferred method, but enzyme-linked immunoabsorbent assay and polymerase chain reaction methods are now available.
- In falciparum malaria, signet-ring forms are most abundant on peripheral smear immediately after a fever spike.
Because parasites can be absent between attacks, the blood must be examined on 3 to 4 successive days before malaria can be ruled out. Presence of pigment in peripheral monocytes or neutrophils should encourage a continued search for parasites. Thin smears need to be examined for at least 15 minutes using a high-power oil objective microscope (1000X magnification).
Thick smears are the most reliable method for detecting malaria. A 5-minute search will generally yield the diagnosis.
The clinician’s primary goal is to differentiate potentially fatal P. falciparum from other, more benign forms of malaria. For this purpose, three new assays have been developed: an enzyme-linked immunoabsorbent assay (ELISA) for histidine-rich P. falciparum antigen, an immunoassay for species-specific parasite lactic dehydrogenase isoenzymes, and polymerase chain reaction amplification of parasite Deoxyribonucleic acid or mRNA. Anemia, elevated levels of lactic dehydrogenase, and increased reticulocytes are associated with red blood cell hemolysis.
An elevated unconjugated bilirubin level without a significant increase in hepatic enzymes is also observed when hemolysis is severe. A reduced white blood cell count is noted in a high percentage of patients, and thrombocytopenia is common. Elevated serum creatinine, proteinuria, and hemoglobinuria are found in severe cases of P. falciparum. Hypoglycemia may also complicate severe cases of P. falciparum, requiring close monitoring of blood sugars during the acute illness.
Prophylaxis and Treatment
Drug treatment exploits unique targets in the parasite not found in host cells. The aminoquinolones, chloroquine, quinine, mefloquine, primaquine, and halofantrine inhibit proteolysis of hemoglobin in the food vacuole and inhibit the heme polymerase that Plas-modium requires for production of malaria pigment. Inhibition of these functions kills the organism. Pyrim ethamine, sulfonamides, and dapsone are folate antagonist. Atovaquone inhibits parasite mitochondrial transport. Artemisinin derivatives bind iron in the malarial pigment to produce free radicals that damage parasite proteins.
These derivatives are faster-acting than quinine, and they have activity against all stages of the intraerythrocytic life cycle. In recent years, many areas of Africa, northern South America, India, and Southeast Asia have become populated with chloroquine-resistant P. falciparum. These strains contain an energy-dependent chloroquine efflux mechanism that prevents the drug from concentrating in the parasite. Resistance to mefloquine and halofantrine has also developed, being seen primarily in Southeast Asia. Chemoprophylaxis should start 2 weeks before departure to an endemic area and continue until 4 weeks after return.
Because of the continual changes in resistance patterns, up-to-date prophylactic and treatment regimens should be reviewed at the CDC’s Web site (www.cdc.gov/travel). For areas with chloroquine-suscepti-ble P. falciparum, chloroquine is the drug of choice.
The adult dosage is 300 mg base (500 mg of chloroquine phosphate) orally per week. In areas of chloroquine-resistance, mefloquine 250 mg (228 mg base) orally per week, or doxycycline 100 mg orally per day, or primaquine 0.5 mg/kg base per day, or atovaquone 250 mg combined with proguanil 100 mg orally per day (in a combination tablet called Malarone), or chloroquine at the formerly mentioned dose combined with proguanil 200 mg per day.
A vaccine is not available, and the immune response required to protect the host against malaria is poorly understood, making development of an effective vaccine a formidable task. All individuals without previous immunity who contract falciparum malaria should be hospitalized, because their clinical course can be unpredictable.
About Malaria Prophylaxis
- Determine if the traveler will be visiting areas with chloroquine-resistant strains (check www.cdc.org/travel).
- Begin prophylaxis 2 weeks before travel to insure that no intolerable side effects develop.
- Continue prophylaxis for 4 weeks after return.
Patients with the P. vivax, P. ovale, and P. malariae strains can usually be treated as outpatients if follow-up will be reliable.
The treatment for these three strains and for chloroquine-sus-ceptible P. falciparum is the same: an initial dose of oral chloroquine 600 mg base (1000 mg chloroquine phosphate), followed 6 hours later by 300 mg base (500 mg phosphate), repeated on days 2 and 3. To prevent relapse of P. vivax or P. ovale, these infections also require treatment with oral primaquine 15.3 mg phosphate base (26.5 mg phosphate salt) daily for 14 days, or 45 mg base (79 mg salt) weekly for 8 weeks.
This agent kills dormant hepatic hypnozoites, preventing their subsequent development into infective schizonts. Before the primaquine is administered, the patient should be tested for glucose-6-phosphate dehydrogenase deficiency, because patients with this deficiency are at risk of severe hemolysis during primaquine treatment. Given the worldwide prevalence of chloroquine resistance, unless absolute assurance can be obtained that travel was only in regions with chloroquine-sensitive P. falciparum, patients should be presumed to have a resistant strain.
Treatment of chloroquine-resistant P. falciparum is evolving and has become complex. Although artemisinin derivatives are not currently available in the United State, they have shown superior efficacy for severe chloroquine-resistant P. falciparum infection. They also reduce gametocyte carriage. Their use therefore decreases infectivity after treatment, and they can eliminate malaria transmission in endemic areas.
These agents are short-acting, and they should be combined with one or more other classes of antimalarial agents. Dihydroartemisinin (6.3 mg/kg daily) combined with piperaquine (50 mg/kg daily) has produced superior response rates; however, trials of various combinations are ongoing. Monotherapy is discouraged because of the rapid development of resistance.
Artemisinin manufacturing quality is not currently reliable, and these agents are therefore not recommended as standard therapy. In the United States, quinine 650 mg every 8 hours for 3-7 days, plus doxycycline 100 mg twice daily for 7 days remains the recommended regimen.
Atovaquone-proguanil (250 mg/100 mg tablets) four times daily for 3 days is equally efficacious. A single high dose of mefloquine (1250 mg) alone has been recommended as alternative therapy; however, this treatment frequently causes intolerable side effects, including vertigo (10% to 20%), gastrointestinal disturbances, seizures, and (less commonly) psychosis. In addition, mefloquine-resistant P. falciparum is increasing in frequency. If a patient is too ill to take oral medicines, intravenous quinidine is the treatment of choice.
This drug is three to four times more active than is intravenous quinine, and serum levels can be measured. Furthermore, parenteral quinine is no longer available in the United States. Quinidine gluconate salt 10 mg/kg loading dose (maximum 600 mg) in normal saline should be infused slowly over 1 to 2 hours, followed by a continuous infusion of 0.02 mg/kg every minute until the patient is able to take oral medication. Given the rapid changes in malaria resistance patterns and newly reported clinical trials, health care providers should refer to excellent Web sites operated by recognized authorities that outline up-to-date treatment regimens.
About Choosing Chemotherapy for Plasmodium Infection
- Determine whether the traveler came from a chloroquine-resistant area: a) For chloroquine-sensitive strains, use chloro-quine. b) For resistant strains, use quinine or an equivalent regimen. c) Artemisinin derivatives have improved efficacy for severe disease, but manufacturing quality is unreliable. Monotherapy is discouraged. Not available in the United Kingdom or the United States.
- Determine whether the patient is too ill to take oral medicines (requires intravenous quinidine).
- Determine whether the patient has Plasmodium vivax or ovale (requires primaquine, if not deficient in glucose-6-phosphate dehydrogenase).
- Referto Web sites run by health authorities forthe most current antimalarial regimens.
About Managing Patients with Plasmodium falciparum
- Levels of parasitemia above 5% constitute a medical emergency and require immediate institution of antimalarial treatment.
- Hematocrit, blood sugar, volume status, cardiac rhythm, renal function, central nervous system function, and arterial oxygenation must all be closely monitored.
- In the nonimmune host, the course of P. fakiparum infection is not predictable.
- The severity of organ damage and risk of death correlate with the level of parasitemia.
The risk of end-organ damage and death increases with the patient’s level of parasitemia. Levels above 5% constitute a medical emergency, and patients with these levels require intensive treatment. Patients with no immunity and levels of P. fakiparum parasitemia above 10% to 15% should be considered for exchange transfusion, a measure that can be life-saving.
However, patients with levels of parasitemia of greater than 50% have survived without blood exchange.
Volume status, renal function, and serum glucose must be carefully monitored. Respirator support may be required in cases of severe pulmonary edema. Intravenous steroids have been shown to be harmful in cases of cerebral malaria, and those agents should therefore be avoided. Because of the risk of arrhythmias associated with quinine, quinidine, mefloquine, and halofantrine, cardiac function should be monitored in patients treated with those agents.
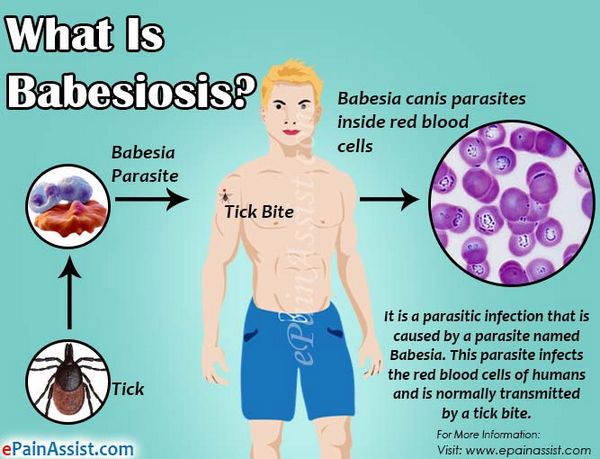
Babesiosis
Potential Severity
Usually causes mild disease, but in splenectomized patients can be fatal.
Prevalence, Epidemiology, and Life Cycle
Babesiosis was once thought to be a disease only of cattle and wild animals. However, in the last 30 years this organism has been found to occasionally infect humans. More than 100 cases of human babesiosis have been described, many occurring in Massachusetts on the islands of Nantucket and Martha’s Vineyard.
Other cases have been described throughout New England, New York, Maryland, Virginia, Georgia, Wisconsin, Minnesota, Washington State, and California. Like malaria, Babesia is a blood protozoan. It has a lifecycle similar to that of Plasmodium; however, Babesia is transmitted by the deer tick, Ixodes scapularis. Curiously, Babesia do not infect deer. However, the intermediate host, the white-footed deer mouse, is readily infected by Babesia microti, the primary strain causing human disease in the United States. In endemic areas, the percentage of these rodents infected by Babesia can reach 60%.
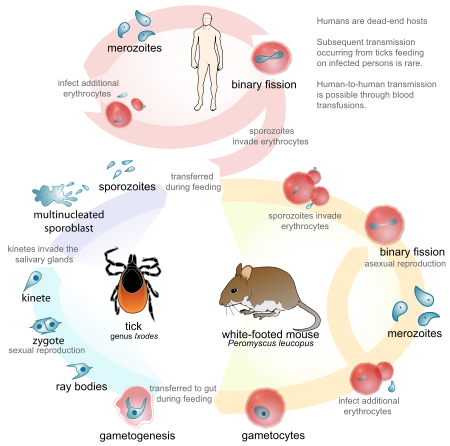
During its larval and nymph phases, the tick lives on the deer mouse, where it obtains blood meals. The nymph can leave the deer mouse and attach to humans. After attachment, this tiny tick (2 mm in diameter) eats a blood meal and introduces the Babesia sporozoite. The sporozoites enter human red blood cells. The mature signet-ring-shaped trophozoite multiplies asexually by binary fission, forming characteristic tetrads. Subsequently, it lyses the host red blood cell. Because multiplication is asynchronous, massive hemolysis is not seen. Also, unlike Plasmodium, Babesia lacks a hepatic phase.
The rise in the incidence of babesiosis has been attributed to the decreased popularity of deer hunting and the associated increase in deer and deer tick populations. Also, migration to the suburbs in the United States has brought humans in closer proximity to the mouse reservoirs harboring the infectious Ixodes scapularis nymph. The infection is contracted by humans during the months of May through September when the nymphs are feeding.
About the Babesia Lifecycle
- The small nymph form (2 mm in diameter) of the deer tick, Ixodes scapularis, carries Babesia from white deer mice to humans.
- In human red blood cells, the mature signet-ring trophozoite multiplies by binary fission forming characteristic tetrads.
- Multiplication is asynchronous, and therefore hemolysis is never massive.
- Babesiosis has no hepatic phase.
About Babesia Epidemiology
- Endemic in areas where the deer population is abundant.
- Requires the presence of the white deer mouse, which harbors the infectious deer tick (Ixodes scapularis) nymphs.
- Human infections occur during the period of nymph feeding (May to September).
Clinical Presentation
Case 2
A 65-year-old white female presented with intermittent fever for the preceding 2 months, associated with inter-mitten t myalgias and fatigue. She had jus t returned from a 2-month summer vacation in Martha’s Vineyard, Massachusetts. She denied any history of tick bites. One month earlier, she had been diagnosed with Lyme disease. However, despite appropriate treatment, her fevers did not resolve. Aside from a mild anemia, her routine blood tests were normal; however, Giemsa stain of her peripheral blood revealed occasional red blood cells containing ring forms, some in tetrads. Treatment with clindamycin and quinine caused a rapid resolution of her fever. The symptoms of babesiosis are nonspecific, making the disease difficult to diagnose clinically.
Generally, patients present 1 to 3 weeks after exposure with a flu-like illness. Fever, chills, myalgias, arthralgias, fatigue, and anorexia are most common. The illness presents during the summer months as a “summer flu.” In endemic areas, the clinician should inquire about recent hiking in tick-infested locations, particularly those with tall grasses and brush. Patients often do not give a history of tick bites, having failed to detect the attached nymph because of its small size (the diameter of a small freckle). In the normal host, the disease may cause minimal symptoms and resolve spontaneously. However, in older patients or in those who have undergone splenectomy, infection can be more severe and persistent.
Cases of adult respiratory distress syndrome and hypotension have been reported, and on rare occasions, patients have died. In Europe, cases have strictly involved splenectomized patients, and the clinical presentation has been more fulminant, being associated with severe hemolysis and death. Patients with babesiosis may also have symptoms suggestive of Lyme disease, particularly the skin rash of erythema migrans. Ixodes scapularis is also the vector for Borrelia burgdorferi, and in one series of cases, 54% of patients with babesiosis also had antibodies against the Lyme spirochete, suggesting that these patients had dual infections.
About the Clinical Presentation of Babesiosis
- Presents as the “summer flu” 1 to 3 weeks after exposure.
- History of hiking in tick-infested areas.
- Often no history of tick bite, because the Ixodes scapularis nymph is mistaken for a small freckle.
- More serious disease occurs in splenectomized patients and elderly individuals.
- Patients with babesiosis may also have Lyme disease, because Ixodes scapularis transmits both infections.
Diagnosis and Treatment
Giemsa stain of thick and thin smears from the peripheral blood should be examined under an oil-immersion objective. Small ring forms, often grouped in tetrads are the only form seen. Babesiosis is frequently mistaken for P. falciparum.
The classic tetrad is not observed in Plasmodium infection, and the banana-shaped gametocytes observed in P. falciparum are never observed in Babesia. An indirect immunofiu-orescence antibody titer that measures antibody against B. microti, the primary form that causes babesiosis in the United States, is available through the CDC.
Significant increases in antibody titer develop 3 to 4 weeks after the infection is contracted. Treatment should be initiated in splenectomized patients and in other patients with serious disease. Clindamycin combined with oral quinine is the preferred regimen. Another equally effective regimen is azithromycin and atovaquone. This combination is associated with fewer adverse reactions than is clindamycin and quinine.
Chloroquine, often initiated when Babesia is mistaken for P. falciparum, is not effective. Similarly doxycycline, pentamidine, primaquine, and pyrimethamine-sulfadoxine (Fansidar) are not efficacious.
About Diagnosis and Treatment of Babesiosis
- Giemsa stain of the peripheral blood remains the best way to make the diagnosis.
- Only ring forms are seen.
- Frequently mistaken for Plasmodium falciparum.
- Tetrad ring forms strongly support the diagnosis of babesiosis.
- Many malaria regimens, including chloroquine and primaquine,are not effective in babesiosis.
Table. Antiparastic Therapy Dosingh
| Parasite | Preferred therapy | Alternative therapy | ||
| Babesia | ||||
| Intravenous clindamycin 1.2 gq12h,0R Clindamycin 600 mg q8h for 7-10 days, AND quinine 650 mg q8h for 7-10 days | Atovaquone 750 mg q12h for 7-10 days, AND azithromycin 600 mg daily for 7-10 days | |||
| Leishmania | ||||
| Visceral | Liposomal amphotericin В 3 mg/kg daily on days 1-5,14,21 | Intravenous or intramuscular sodium stibogluconate 20 цд/кд daily for 28 days | ||
| Cutaneous | Intravenous or intramuscular sodium stibogluconate 20 mg/kg daily for | Intracutaneous sodium stibogluconate daily for 21 days Liposomal amphotericin В for unresponsive lesions | ||
| Trypanosoma cruzi (Chagas’ disease) | ||||
| Nifurtimox 8-10 mg/kg daily divided q6h for 90-120 days | Benznidazole 5 mg/kg daily for 60 days | |||
| Trichuris (whip worm) | ||||
| Mebendazole 100 mg q12h for 3 days, OR Albendazole 400 mg q24h for 3 days | Ivermectin 200ug/kg daily for 3 days, OR Nitazoxanide500mg q12h for 3 days | |||
| Ascaris | ||||
| Mebendazole 100mgq12h for3 days | Pyrantel pamoate 11 mg/kg (maximum 1 g) q12h for 3 days, OR Albendazole 400 mg once, OR Nitazoxanide 500 mg ql 2h for 3 days | |||
| Enterobius (pin worm) | ||||
| Mebendazole 100 mg once,OR Albendazole 400 mg once, OR Pyrantel pamoate 11 mg/kg (maximum 1 g) once Repeat selected treatment after 2 weeks | ||||
| Strongyloides | ||||
| Ivermectin 200 цд/kg daily for 2 days For disseminated disease, continue for 7 days (longer if immunocompromised) | Albendazole 400 mg q24h for 3 days | |||
| Hook worm | ||||
| Albendazole 400 mg once, OR Mebendazole lOOmg q12h for 3 days, OR Pyrantel pamoate 11 mg/kg (maximum 1 g) for 3 days | ||||
| Trichinella | ||||
| Steroids for severe symptoms, PLUS mebendazole 200-400 mg q8h for 3 days, THEN 400-500 mg q8h for 10 days | Albendazole 400 mg ql2h for 8-14 days | |||
| Echinococcus granulosus (hytlatid cyst) | ||||
| Aspiration or surgical excision, PLUS perioperative albendazole | Albendazole 400 mg q12h for 1-6 months | |||
| Echinococcus multilocularis | ||||
| Surgical excision or aspiration | ||||
| Taenia solium (cysticercosis) | ||||
| Albendazole 400 mg ql 2h for 8-30 days, repeated as necessary Concurrent steroids for central nervous system disease | Praziquantel 50-100 mg/kg daily divided q8h for 30 days Surgery | |||
| Schistosomes (Schistoseima) | ||||
| S. mansoni S. haematobium | Praziquantel 40 mg/kg divided ql 2h over 1 day Praziquantel as for S. mansoni | Oxamniquine 15 mg/kg once (30 mg/kg once for East Africa; 30 mg/kg q24h for 2 days for Egypt and South Africa) | ||
| S.japonicum and S. mekongi | Praziquantel 60 mg/kg divided q8h over 1 day | |||
| Clonorchis sinensis | ||||
| Praziquantel 75 mg/kg divided q8h over 1 day | ||||
| Fasciola hepatica | ||||
| Triclabendazole 10 mg/kg once | ||||
| Paragonimus westermani | ||||
| Praziquantel 75 mg/kg daily divided q8h for 2 days | Bithionol 30-50 g/kg on alternate days for 10-15 doses | |||
| Wuchereria bancrofti and Brugia malayi | ||||
| Diethylcarbamazine 6 mg/kg once | Doxycycline 100 mg ql2h for 3 weeks before diethylcarbamazine may reduce febrile reactions and kills adult worms | |||
| Onchocerca volvulus | ||||
| Ivermectin 150 µg/kg once Repeat every 6-12 months | ||||
| Loa loa | ||||
| Diethylcarbamazine 6 mg/kg once | ||||
Tissue Protozoa
Intestinal Helminths
Tissue And Blood Helminths
Other, Less Common Tissue Flukes
Other flukes that can infect humans undergo a life cycle similar to that of Schistosoma, requiring snails as the intermediate host. However, rather than gaining entry by penetrating the human skin, the cercariae take up residence in other food sources and become encysted. Infection is contracted when the human host eats cercariae contaminated food.
Clonorchis sinensis (Chinese liver fluke) infections result from the ingestion of raw or undercooked freshwater fish. Infections occur in China, Hong Kong, and Vietnam.
Worms gain entry into biliary tract via the ampulla of Vater. Infection can be complicated by cholangitis and, later, by cholangiocarcinoma. Infections are effectively treated with praziquantel. Fasciola hepatka, another liver fluke, is found in sheep-raising areas of the world, including South America, Australia, China, Africa, and Europe. Ingestion of vegetables contaminated with encysted cercariae is the most common route of infection. This fluke is treated with praziquantel or bithionol.
Paragonimus westermani (lung fluke) is contracted by eating raw or pickled crawfish or freshwater crabs. This parasite is found in Central and South America, West Africa, India, and East Asia. This parasite first enters the gastrointestinal tract and subsequently penetrates through the diaphragm, entering the pleural cavity and lungs, causing respiratory symptoms. Praziquantel is the treatment of choice.
About the Diagnosis and Treatment of Schistosomiasis
- Characteristic eggs in the stool or urine (check between noon and 2 pm) or on tissue biopsy are diagnostic; consider rectal biopsy in Schistosoma mansoni.
- Eggs may not be seen in chronic disease, anti-schistosome antibody may be helpful
- Praziquantel is the treatment of choice.
Filariasis (Wuchereria Bancrofti And Brugia Malayi)
Dirofilariasis (Dog He Art Worm)
Humans are an accidental host in dirofilariasis. The disease is most commonly found in the southeastern United States and is transmitted by mosquitoes. After developing in the subcutaneous tissue, the young adult filaria migrate. In dogs, they migrate to the right side of the heart and right pulmonary vessels, where they survive. In humans, they migrate to the lung, but fail to develop. Their deaths produce local granulomatous inflammation. Most human cases present as an asymptomatic pulmonary coin lesion mimicking an early neoplasm. Microscopic examination of the lung biopsy reveals a dead worm. Treatment of human cases is not necessary.
Onchocerciasis
The Onchocerca volvulus parasite is found primarily in Africa, where it infects approximately 20 million people. Cases are occasionally seen in Central and South America. The infection is transmitted by a black fly that swarms around the face, often biting around the eyes and depositing Onchocerca larvae onto the skin.
These larvae penetrate and crawl through the skin and connective tissue. The worms initially cause an itchy erythematous rash. Later, fibrous skin nodules develop. Worms often migrate into the anterior chamber of the eye, causing inflammation and blindness.
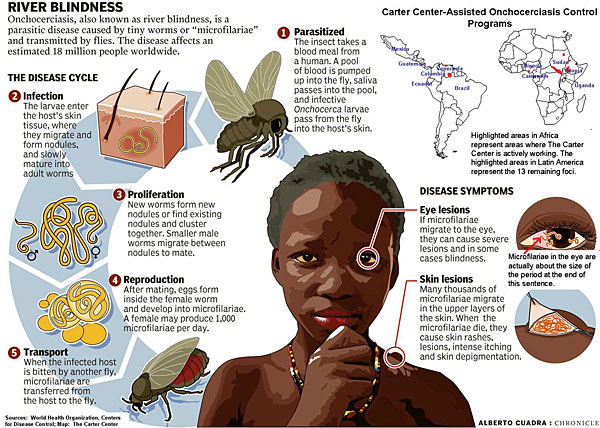
Because the offending black fly is commonly found near streams, this disease has been called “river blindness.” The diagnosis is made by skin snips or by visualizing worms in a slit lamp examination of the eyes. The treatment of choice is a single dose of ivermectin repeated at 3-month intervals until symptoms resolve. Fever, itching, and an urticarial rash may develop as result of dying microfilaria.
Loiasis
The loa loa microfilaria is also transmitted by a fly and the disease is found in Western and Central Africa. The microfilaria migrate through the skin, causing localized edema called Calabar swellings. Several hours before swelling occurs, local itching and pain are noted.
Occasionally the microfilaria can be seen migrating through the subconjuctiva, causing intense conjunctivitis. Active microfilaria migration is associated with marked peripheral eosinophilia.
The diagnosis is made by daytime blood smear. Diethylcarbamazine or ivermectin are recommended as treatment. Diethylcarbamazine can precipitate encephalitis in heavily infected patients.





