Amantadine (Symmetrel) is an antiviral medication primarily used to treat and prevent influenza A infections. However, its effectiveness has diminished due to widespread resistance. Additionally, it is employed in managing Parkinson’s disease symptoms and drug-induced movement disorders by increasing dopamine levels in the brain. Amantadine is available in various forms, including tablets and extended-release capsules. Symmetrel is a dopaminergic drug, which means it can increase the levels of certain chemicals that transmit impulses in the nervous system, including the brain.
Uses
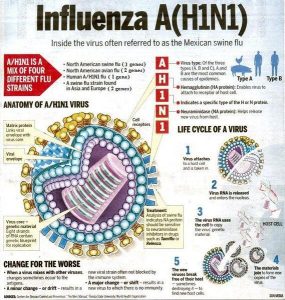
Amantadine is an antiviral medication that is 70-90% effective in preventing influenza A infections and can reduce symptoms when administered within 24-48 hours of onset. While it is comparable to rimantadine and vaccination, its effectiveness diminishes after 48 hours, and resistance among influenza strains is a growing concern. Additionally, Amantadine is primarily used for influenza A.
Symmetrel capsules are used:
- to treat Parkinson’s disease by improving muscle control and reducing stiffness, shakiness and shuffling;
- in the treatment of shingles (herpes zoster) to reduce pain.
Symptoms
Certain signs can help differentiate the causes of influenza-like illness. Nasal congestion and rhinorrhea are common in most cases but rare in inhalational anthrax.
Fever, chills, fatigue, cough, headache, myalgia, sore throat, and rhinorrhea occur in 64-94% of laboratory-confirmed influenza cases. In contrast, similar symptoms appear in 62-94% of patients with other viral or bacterial infections (excluding inhalational anthrax), though fever is present in only 40-73%. Nausea or vomiting occurs in 12%, abdominal pain in 22%, shortness of breath in 6%, and chest discomfort in 23-35% of these patients. In inhalational anthrax cases, 60-80% experience shortness of breath and chest pain, 80-90% have nausea, and 70% report drenching sweats.
Initial chest radiographs of inhalational anthrax patients often show mediastinal widening, infiltrates, and pleural effusion. While most influenza-like illnesses do not show pneumonia on radiographs, this can occur in young children, senior patients, or those with chronic lung diseases. Influenza-associated pneumonia affects approximately 1-5% of adults and over 20% of older adults infected with influenza.
Mechanism of Action
The exact mechanism of the antiviral activity of Amantadine has not been fully elucidated.
Amantadine, like rimantadine, inhibits viral replication by interfering with the influenza A virus M2 protein, an integral membrane protein. The M2 protein of influenza A functions as an ion channel and is essential in at least two aspects of virus replication: disassembly of the infecting virus particle and regulation of the ionic environment of the transport pathway. By interfering with the ion channel function of the M2 protein, Amantadine inhibits two stages in the replicative cycle of influenza A. Early in the virus replicative cycle, Amantadine inhibits the uncoating of the virus particle, presumably by inhibiting the acid-mediated dissociation of the virion nucleic acid and proteins, which prevents nuclear transport of viral genome material.
Amantadine also prevents viral maturation in some strains of influenza A (e.g., H7 strains) by promoting pH-induced conformational changes in influenza A hemagglutinin during its intracellular transport late in the replicative cycle. The virus’s adsorption and penetration into cells do not appear to be affected by Amantadine. In addition, Amantadine does not interfere with the synthesis of viral components (e.g., RNA-directed RNA polymerase activity).
Amantadine treatment of established influenza A infection does not appear to interfere with antibody response to the infection; however, some reduction in local immune responses has been observed in some patients. Because prophylactic use of Amantadine can prevent influenza illness and, to a lesser extent, subclinical infection, some individuals who take Amantadine can still develop immune responses that may protect them when they are exposed to the same or antigenically related viruses following discontinuance of amantadine prophylaxis. Amantadine does not interfere with the immunogenicity of influenza A virus vaccine.
Amantadine-mediated increases in lysosomal pH may inhibit virus-induced membrane fusion in enveloped RNA viruses susceptible to higher concentrations of Amantadine than those required to inhibit influenza A.
Pharmacokinetics
Absorption
Amantadine hydrochloride is well absorbed from the gastrointestinal tract, with peak blood concentrations occurring 1-4 hours after an oral dose. For a 100 mg capsule, average peak plasma concentrations are around 0.22 mcg/mL at 3.3 hours, and similar concentrations are observed for the oral solution. Higher doses can lead to greater than proportional increases in plasma concentration, particularly beyond 200 mg daily.
Distribution
Amantadine is distributed into various tissues, including the heart, lungs, and kidneys, with higher concentrations in lung tissue compared to blood. It is also found in nasal secretions and breast milk, with a notable erythrocyte-to-plasma ratio. The volume of distribution is approximately 3-8 L/kg.
Elimination
The elimination half-life of Amantadine averages around 24 hours. Still, it can be prolonged in geriatric patients and those with renal impairment. It is primarily excreted unchanged in urine, with some metabolites identified. Urine acidification can increase amantadine excretion, while hemodialysis removes minimal amounts of the drug.
Ingredients
The active ingredient in this medicine is amantadine hydrochloride. Each Symmetrel capsule contains 100 mg amantadine hydrochloride. The other ingredients are lactose, povidone, magnesium stearate, red iron oxide (E172), titanium dioxide (E171), gelatin, and white printer ink.
Dosage
Symmetrel capsules are brownish-red, hard gelatine capsules with SYMM printed on them in white. Symmetrel capsules come in boxes of 56 capsules.
Amantadine hydrochloride is administered orally as a single daily dose or, preferably, in 2 equally divided doses to minimize transitory adverse effects. If insomnia occurs, the last daily dose should be taken several hours before retiring.
| Population | Dosage |
|---|---|
| Adults (18-64 years) | 200 mg daily (single dose or 100 mg twice daily); maximum 100 mg daily for prophylaxis in some cases. |
| Geriatric Patients (65+ years) | 100 mg once daily; may need further reduction based on renal function. |
| Children (9-12 years) | 100 mg twice daily or 200 mg daily (in 1 or 2 divided doses). |
| Children (1-9 years) | 4.4-8.8 mg/kg daily (up to 150 mg max), given in 1 or 2 divided doses. |
| Children (<1 year) | Dosage not established; use must be determined by a doctor. |
Duration of Therapy
Treatment
In the symptomatic treatment of respiratory tract illness caused by influenza A virus, amantadine hydrochloride should be administered as soon as possible, preferably within 24-48 hours after the onset of symptoms. The ACIP currently states that it may be advisable to discontinue amantadine treatment as soon as clinically warranted, generally within 3-5 days or 24-48 hours after symptoms disappear, since there is some risk that strains of influenza A resistant to Amantadine and rimantadine may emerge during treatment with the drug. However, immunocompromised individuals may require a longer course of therapy.
Prevention
When a presumed influenza A outbreak occurs in a hospital, nursing home, or other institution housing high-risk patients, amantadine prophylaxis should be started as soon as possible after recognition of the outbreak and continued at least 2 weeks or until approximately one week after the end of the outbreak. When amantadine hydrochloride is used as an adjunct to the influenza virus vaccine, the drug is usually administered for 2 weeks after the vaccine is given to provide chemoprophylaxis until a protective antibody response develops. Children younger than 9 years of age receiving influenza virus vaccine for the first time may require amantadine prophylaxis for up to 6 weeks following vaccination or until 2 weeks after the second dose of vaccine.
When vaccination is contraindicated, amantadine prophylaxis may be given throughout the local influenza A outbreak, which may be as long as 6-12 weeks. Amantadine prophylaxis can also be given throughout the period of outbreak when poor antibody response to influenza vaccine is expected (e.g., in patients with severe immunodeficiency, including acquired immunodeficiency syndrome [AIDS]).
The manufacturer states that prophylaxis with amantadine hydrochloride should be instituted before or as soon as possible after the patient has contact with an individual having a respiratory illness thought to be caused by influenza A virus, and should be continued for at least 10 days following a known exposure.
Dosage in Renal Impairment
In patients with renal impairment, amantadine hydrochloride dosage should be carefully adjusted, and some clinicians recommend that blood concentrations of the drug be monitored frequently. One manufacturer recommends that patients with creatinine clearances of 15-50 mL/minute per 1.73 m receive 200 mg of Amantadine on the first day, followed by 100-mg maintenance doses given once daily in patients with creatinine clearances of 30-50 mL/minute per 1.73 m or once every other day in those with creatinine clearances of 15-29 mL/minute per 1.73 m. This manufacturer recommends that patients with creatinine clearances less than 15 mL/minute per 1.73 m and hemodialysis patients receive 200 mg of Amantadine every 7 days.
Because dosage adjustment based on creatinine clearance may provide only an approximation of the optimal dosage for a given patient, such patients should be observed so that adverse reactions can be recognized promptly and either the dose can be reduced further, or the drug can be discontinued as necessary. Hemodialysis contributes minimally to the clearance of Amantadine.
If You Forget to Take Symmetrel 100mg Capsules
If you miss a dose, take another as soon as you remember unless it is almost time for your next dose. Then go on as before. Do not take a double dose.
If You Stop Taking Symmetrel Capsules
Do not stop taking Symmetrel 100mg capsules suddenly, as your symptoms may worsen.
If you want to stop taking Symmetrel 100mg capsules, ask your doctor, who will tell you how to reduce the dose gradually.
If you are taking antidepressants (used to treat mental disturbances) and you suddenly stop taking Symmetrel capsules, you may develop some symptoms, including:
- fever;
- sweating;
- a rapid heartbeat;
- muscle stiffness (difficulty in movement);
- loss of bladder control (you may have a sudden urge to pass water).
You should contact your doctor immediately if you develop any of these symptoms.
Some patients may notice that this medicine loses its effect after taking it regularly for a few months. If you notice this, tell your doctor.
Ask your doctor or pharmacist if you have any further questions about using this product.
Important Safety Information
Be careful when drinking alcohol while taking Symmetrel 100mg capsules; it may affect you more than usual.
Symmetrel 100mg capsules should be taken with a glass of water.
Symmetrel 100mg Capsules contain lactose. If your doctor has told you that you are intolerant to some sugars, contact your doctor before taking Symmetrel 100mg capsules.
Pregnancy and Breastfeeding
Do not take Symmetrel 100mg capsules if you are pregnant or trying to become pregnant. Do not take Symmetrel 100mg capsules if you are breastfeeding because Symmetrel passes into breast milk and could harm your baby. Ask your doctor or pharmacist for advice before taking any medicine.
Driving and Using Machines
Taking Symmetrel capsules may make your vision blurred or make you feel dizzy. If you are affected, you should not drive or use machines until the effect has worn off.
Resistant Strains of Influenza A Virus
Amantadine- and rimantadine-resistant strains of influenza A can develop in about 33% of patients treated with these antivirals. Patients may initially shed susceptible virus strains but can start shedding resistant strains after 2-7 days of therapy, with immunocompromised individuals potentially shedding them for longer.
To limit resistance, amantadine treatment should be stopped after 3-5 days or once symptoms resolve. Although most patients recover even after resistant strains emerge, these strains are still pathogenic. They can hinder drug prophylaxis for close contacts. Individuals with influenza-like symptoms should avoid contact with uninfected individuals, regardless of antiviral treatment.
Pediatric Precautions
The safety and efficacy of Amantadine in children younger than 1 year of age have not been established. When used in children, Amantadine has caused CNS symptoms, which resolved when the drug was discontinued. The incidence of adverse CNS-related effects appears to be higher in individuals receiving Amantadine than in those receiving rimantadine. An increased incidence of seizures has been reported in children with an underlying seizure disorder receiving Amantadine.
Geriatric Precautions
While the safety and efficacy of Amantadine in geriatric patients have not been explicitly established, the drug has been used in many geriatric patients. The frequency and severity of adverse CNS effects reported in individuals older than 65 years of age receiving Amantadine are higher than those reported in geriatric individuals receiving rimantadine. Geriatric adults may have decreased renal function, and because individuals with renal impairment may be at increased risk of amantadine-induced toxicity, the dosage of amantadine hydrochloride for adults in this age group should not exceed 100 mg daily. This dosage may need to be reduced further in some geriatric patients.
Mutagenicity and Carcinogenicity
Amantadine was not mutagenic in the Ames microbial test using Salmonella typhimurium or a mammalian mutagen assay using Chinese hamster ovary cells when the tests were performed with or without metabolic activation. In addition, there was no evidence of chromosome damage in an in vitro test using freshly derived and stimulated human peripheral blood lymphocytes (with or without metabolic activation) or an in vivo mouse bone marrow micronucleus test (140-550 mg/kg; estimated human equivalent dosage of 11.7-45.8 mg/kg based on body surface area conversion). Long-term animal studies have not been performed to evaluate the carcinogenic potential of Amantadine.
HIV-Infected Individuals
The ACIP, the Committee on Infectious Diseases of the American Academy of Pediatrics (AAP), and the Prevention of Opportunistic Infections Working Group of the US Public Health Service and the Infectious Diseases Society of America (USPHS/IDSA) currently recommend that annual vaccination be considered for all HIV-infected adults and children 6 months of age or older since these individuals may be at high risk of complications from influenza.
Influenza virus is not traditionally classified as an opportunistic pathogen. Still, many experts consider vaccination against the virus as logical in any HIV-infected individual (whether symptomatic or asymptomatic) because of the possible risks of respiratory infections in such patients, and protective antibody levels are likely in many such vaccines.
Antiviral prophylaxis may be used in conjunction with, or as an alternative to, influenza virus vaccine in HIV-infected individuals who may have a poor antibody response to the vaccine and/or high risk of exposure to influenza A, especially during influenza epidemics or institutional outbreaks. The USPHS/IDSA recommends oseltamivir to prevent influenza A and B virus infection and rimantadine or Amantadine to prevent influenza A infection.
Contraindications
Amantadine should be used cautiously in patients with liver disease, uncontrolled psychosis, seizure disorders, or those on CNS-active medications. Patients with a history of seizures require close monitoring for increased activity. Due to potential CNS effects and visual disturbances, Amantadine may impair the ability to perform tasks requiring alertness, such as driving. It is contraindicated in individuals with untreated angle-closure glaucoma.
Neuroleptic malignant syndrome (NMS) has been reported, particularly when Amantadine is reduced or withdrawn, necessitating careful observation in patients on antipsychotics. Dosage adjustments may be needed for those with renal impairment, congestive heart failure, or orthostatic hypotension. Resistant strains of influenza A can emerge during treatment, posing a risk of transmission to high-risk individuals.
Individuals with influenza-like illness should minimize contact with uninfected persons. Clinicians should consider potential bacterial infections when treating suspected influenza cases. Amantadine is contraindicated in patients with known hypersensitivity to adamantane derivatives.
Do not take Symmetrel 100mg capsules in the following cases:
- if you are allergic (hypersensitive) to amantadine hydrochloride or any of the ingredients of Symmetrel capsules (see Section 6 Further information);
- if you suffer from fits (convulsions), for example, epilepsy;
- if you have ever had an ulcer in your stomach or small intestine,
- if you suffer from any severe kidney disease;
- if you are pregnant or trying to become pregnant;
- if you are breastfeeding.
If any of the above applies to you, or if you need more clarification, speak to your doctor or pharmacist before you take Symmetrel capsules.
Before you take Symmetrel capsules, tell your doctor if:
- you suffer from any liver or kidney disease;
- you have a history of disease involving the heart and blood vessels;
- you are currently suffering from heart problems or heart failure (heart problems that cause shortness of breath or ankle swelling);
- you have any mental illness, for example, schizophrenia or dementia;
- you have increased pressure in the eyes (glaucoma).
If any of the above applies to you, or if you need more clarification, speak to your doctor or pharmacist before you take Symmetrel capsules.
Interactions
Tell your doctor or pharmacist if you are taking or have recently taken any of the following medicines, as they may interact with Symmetrel capsules:
| Anticholinergics | such as procyclidine, used to treat Parkinson’s disease |
| Antispasmodics | such as hyoscine, used to treat stomach spasms or cramps |
| Levodopa | used to treat Parkinson’s disease |
| Antidepressants | such as chlorpromazine, haloperidol, used to improve thoughts, feelings and behaviour when these are disturbed in certain medical conditions |
| Diuretics | such as hydrochlorothiazide, amiloride or triamterene, used to relieve water retention and reduce high blood pressure. |
Also, this medicine may interact with the following:
- Influenza Virus Vaccine: Amantadine does not interfere with the antibody response to influenza vaccines and can be administered simultaneously.
- CNS Stimulants: Caution is advised when combining Amantadine with CNS stimulants due to the risk of additive effects.
- Co-trimoxazole: Co-administration may reduce Amantadine’s renal clearance, mainly due to trimethoprim. Toxic delirium has been reported following this combination.
- Other Drugs: Combining Amantadine with triamterene and hydrochlorothiazide increased plasma amantadine levels, though the specific cause is unclear. Quinidine or quinine may also reduce Amantadine’s renal clearance. Additionally, using Amantadine with CNS-active antihistamines can increase adverse CNS reactions.
Please tell your doctor or pharmacist if you are taking or have recently taken any other medicines, including medicines obtained without a prescription.
Side Effects
Amantadine can cause a variety of side effects, which can be categorized into several systems:
Common Side Effects
- Gastrointestinal: Nausea (5-10%), anorexia, constipation, diarrhea, dry mouth (1-5%), and vomiting (up to 1%).
- Nervous System: Dizziness, insomnia, nervousness, confusion, agitation, hallucinations, and psychotic episodes. Severe effects may include seizures and neuroleptic malignant syndrome upon withdrawal.
- Cardiovascular: Orthostatic hypotension, peripheral edema (1-5%), congestive heart failure, arrhythmias, and tachycardia.
Less Common Side Effects
- Dermatological: Livedo reticularis (1-5%), rash, and photosensitivity.
- Ocular: Visual disturbances such as corneal opacity and decreased visual acuity (up to 1%).
- Psychiatric: Increased risk of mania and suicidal ideation; symptoms may worsen in patients with a history of mental disorders.
Rare but Serious Effects
- Neurological: Peripheral neuropathy has been reported in long-term users; withdrawal can lead to acute delirium.
- Hematologic: Rare cases of leukopenia and neutropenia.
- Respiratory: Dyspnea and rare occurrences of acute respiratory failure.
The incidence of side effects is often dose-related, with higher doses increasing the risk of severe reactions. Patients with renal impairment or a history of seizures are at greater risk for CNS effects. Regular monitoring is advised for those on long-term amantadine therapy.
Toxicity
Manifestations
Amantadine overdose can lead to fatalities, with the lowest reported lethal dose being 2 g. Symptoms of acute overdosage include cardiac dysfunction (arrhythmias, tachycardia, hypertension), pulmonary edema, renal dysfunction, and CNS toxicity (insomnia, anxiety, confusion, hallucinations). Cases have shown severe reactions such as seizures and hyperthermia. For example, a patient who ingested 2.5 g experienced coma and cardiopulmonary arrest.
Treatment
There is no specific antidote for amantadine overdose. If recent, gastric lavage or emesis may be necessary. Supportive measures include monitoring vital signs and administering fluids. Electrocardiographic monitoring is essential due to the risk of tachyarrhythmias. Acidifying agents can enhance amantadine excretion, but hemodialysis is minimally effective. Observing for hyperactivity and seizures is crucial; sedatives and anticonvulsants may be required. Physostigmine has been used for CNS toxicity management, but its risks should be considered.
Preparations
| Dosage Forms of Amantadine: | |||
|---|---|---|---|
| Amantadine 100 mg tablet | Amantadine HCl 100 mg tablet | Symmetrel 100 mg tablet | Amantadine hcl powder |
| Symmetrel 50 mg/5ml Syrup 480ml Bottle | Pms-Amantadine Hydrochloride 10 mg/ml Syrup | Amantadine HCl 50 mg/5ml Syrup | Mylan-Amantadine 100 mg Capsule |
| Pms-Amantadine Hydrochloride 100 mg Capsule | Amantadine HCl 100 mg capsule | ||
Other Brand Names
1-amino adamantane, Adamantamine, Adamantanamine, Adamantylamine, Amantadine Base, Amantadine HCL, Amantadine Hydrochloride, Amantidine, Aminoadamantane, Gocovri, Dopar.
Storage
Keep out of the reach and sight of children. Do not use Symmetrel capsules after the expiration date stated on the packaging. The expiry date refers to the last day of that month. If your doctor decides to stop your treatment, return any unused medicine to the pharmacist. Only keep it if your doctor tells you to.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines that are no longer required. These measures will help protect the environment.


 (4 votes, average: 4.25 out of 5)
(4 votes, average: 4.25 out of 5)