The same general principles of antiretroviral therapy that apply to HIV-infected adults also apply to HIV-infected pediatric patients; however, the treatment of HIV-infected neonates, children, and adolescents involves unique pharmacologic, virologic, and immunologic considerations. In 1993, the Working Group on Antiretroviral Therapy and Medical Management of HIV-infected Children, a panel convened by the National Pediatric and Family HIV Resource Center (NPHRC), the Health Resources and Services Administration (HRSA), and the National Institutes of Health (NIH) first issued guidelines for the use of antiretroviral agents in the treatment of HIV-infected children. At that time, monotherapy with zidovudine or didanosine was considered an appropriate regimen for initial therapy in HIV-infected pediatric patients.
However, based on studies evaluating multiple-drug antiretroviral regimens in pediatric patients and information regarding the benefits of antiretroviral regimens in HIV-infected adults, the guidelines have been revised several times and now include recommendations for use of multiple-drug antiretroviral regimens for the treatment of pediatric HIV infection. The most recent guidelines for use of antiretrovirals in pediatric HIV infection include information on the diagnosis of HIV in infants; criteria for when to initiate and how to monitor antiretroviral therapy in neonates, children, and adolescents; recommendations for antiretroviral regimens that can be used for initial therapy in pediatric patients; clinical, immunologic, and virologic criteria that can be used to determine when to alter the initial antiretroviral regimen; and information regarding the management of adverse drug reactions in pediatric patients.
Because recommendations for the management of HIV infection in neonates, children, and adolescents are rapidly evolving and increasingly complex, it is recommended that management of HIV-infected pediatric patients be directed by a clinician with expertise in the treatment of pediatric and adolescent HIV infection whenever possible, or that such a specialist be consulted regularly throughout the course of treatment to obtain the most up-to-date information.
Antiretroviral therapy is recommended for all HIV-infected children who have clinical symptoms of HIV infection or evidence of immunosuppression, regardless of age or plasma HIV-1 RNA levels. Ideally, antiretroviral therapy should be initiated in all HIV-infected infants younger than 12 months of age as soon as a confirmed diagnosis is established, regardless of clinical or immunologic status or viral load. There has been some controversy regarding use of antiretrovirals in children 12 months of age or older with asymptomatic HIV infection.
Safety and efficacy of some of the antiretroviral agents in pediatric patients have not been definitely established. However, the absence of results of controlled clinical trials evaluating the drug in pediatric patients should not preclude the use of a commercially available antiretroviral agent when it is indicated in an HIV-infected child, provided current knowledge regarding the drug’s pharmacokinetics and safety are taken into consideration.
Considerable information is available regarding use of NRTIs in pediatric patients. Zidovudine and didanosine have been used extensively in HIV-infected children either alone or in antiretroviral regimens without any unusual adverse effects, and data also are available regarding safety and efficacy of lamivudine in conjunction with zidovudine in children. More limited data are available regarding use of regimens that include stavudine and didanosine, stavudine and lamivudine, or zidovudine and zalcitabine in pediatric patients.
Abacavir, didanosine, lamivudine, stavudine, and zidovudine are labeled by the US Food and Drug Administration (FDA) for use in the treatment of HIV infection in pediatric patients. Zidovudine also is labeled for use in neonates in an attempt to reduce the risk of perinatal transmission of HIV as part of a regimen that includes antepartum and intrapartum zidovudine therapy in the mother and zidovudine therapy in the neonate. While zalcitabine has been used in a limited number of childrenand some information is available regarding safety and efficacy of the drug in pediatric patients, zalcitabine is not currently labeled for use in children younger than 13 years of age.
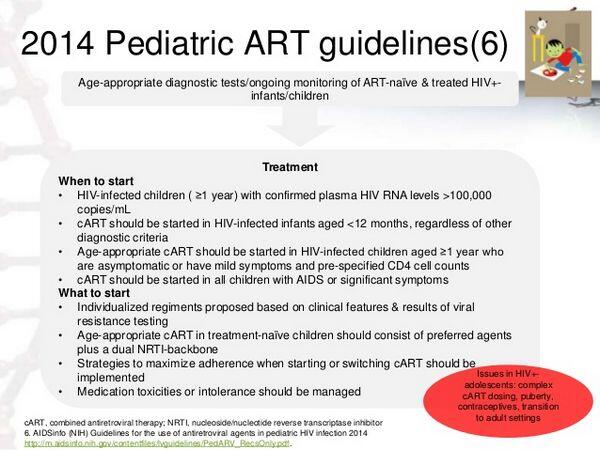
Data are accumulating on safety and efficacy of HIV protease inhibitors in pediatric patients. Both nelfinavir and ritonavir are labeled for use in children 2 years of age or older; amprenavir is labeled for use in children 4 years of age or older. Indinavir and saquinavir have been used in HIV-infected children 3 years of age or older, but safety and efficacy of these HIV protease inhibitors in children or adolescents younger than 16 years of age have not been established. Information regarding use of the NNRTIs in children is limited to date. Nevirapine is labeled for use in pediatric patients 2 months of age or older; efavirenz is labeled for use in children 3 years of age or older; delavirdine is not labeled for use in children younger than 16 years of age.
Initial Antiretroviral Therapy in Treatment-naive Pediatric Patients
The Working Group on Antiviral Therapy and Medical Management of HIV-infected Children recommends aggressive antiretroviral therapy with at least 3 drugs for initial treatment of HIV-infected pediatric patients. The initial antiretroviral regimen selected for infants who were infected perinatally theoretically could be influenced by the antiretroviral regimen their mother may have received during pregnancy, especially if antiretroviral resistance is known or suspected in the mother. However, the Working Group states that currently available data do not suggest that the antiretroviral regimen for infected infants should routinely be chosen based on the regimen used in the mother.
Strongly Recommended Regimens
Based on clinical studies in adults and children, the antiretroviral regimen most strongly recommended for initial therapy in pediatric patients is a 3-drug regimen that includes a highly active HIV protease inhibitor (nelfinavir or ritonavir) and 2 NRTIs (usually zidovudine and didanosine, zidovudine and lamivudine, or stavudine and didanosine; alternatively, stavudine and lamivudine or zidovudine and zalcitabine). A 3-drug regimen that includes efavirenz and 2 NRTIs and a 3-drug regimen of efavirenz, nelfinavir, and one NRTI also are strongly recommended.
Alternative Recommended Regimens
Alternative regimens recommended for initial antiretroviral therapy in pediatric patients include a 3-drug regimen of nevirapine and 2 NRTIs; a 3-drug regimen of abacavir, zidovudine, and lamivudine; a 3-drug regimen of the fixed combination of lopinavir and ritonavir and 2 NRTIs or one NRTI and one NNRTI; or a 3-drug regimen of indinavir or saquinavir (given as liquid-filled [soft gelatin] capsules) and 2 NRTIs. These are considered alternative regimens because durability of suppression of HIV replication may be less than that with strongly recommended regimens (or may not yet be defined), evidence of efficacy may not outweigh potential adverse consequences (toxicity, drug interactions, cost), or experience in infants and children is limited to date.
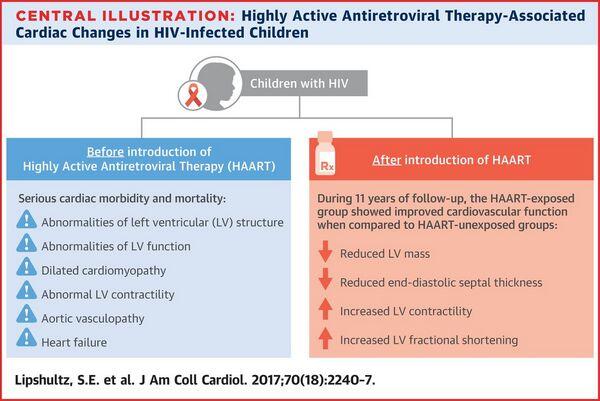
Regimens not Recommended
Based on results of clinical trials in children and adults, monotherapy with any antiretroviral agent or a 2-drug regimen of NRTIs are now considered suboptimal regimens for the treatment of HIV-infected children. Use of zidovudine monotherapy is appropriate only in infants younger than 6 weeks of age when the drug is being used for prevention of perinatal transmission of HIV and the HIV status of the infant is indeterminate. If HIV infection is confirmed, the infant should then be switched to a recommended multiple-drug antiretroviral regimen.
Although use of a 2-drug regimen of 2 NRTIs may be considered in special circumstances (e.g., when the clinician or guardian/patient has concerns regarding the feasibility of adherence to a more complex antiretroviral regimen), results of studies in adults and children indicate that these 2-drug regimens may be less effective in providing durable suppression of HIV replication than 3-drug regimens that also include an antiretroviral agent from another class.
Because data regarding safety and efficacy of amprenavir in children (especially treatment-naive children) are limited to date, the Working Group on Antiretroviral Therapy and Medical Management of HIV-infected Children suggests that use of amprenavir may be considered in previously treated pediatric patients who have failed to respond to regimens that contain other HIV protease inhibitors; however, amprenavir (in conjunction with 2 NRTIs or with abacavir) should be used for initial antiretroviral therapy only in selected pediatric patients and only in special circumstances.

