What Valtrex is and what it is used for
Read indications for use if you want to order Valaciclovir online
Valtrex belongs to a group of medicines called antivirals. It works by killing or stopping the growth of viruses called herpes simplex (HSV), varicella zoster (VZV) and cytomegalovirus (CMV). Valtrex can be used to:
- treat shingles (in adults)
- treat HSV infections of the skin and genital herpes (in adults and adolescents over 12 years old). It is also used to help prevent these infections from returning.
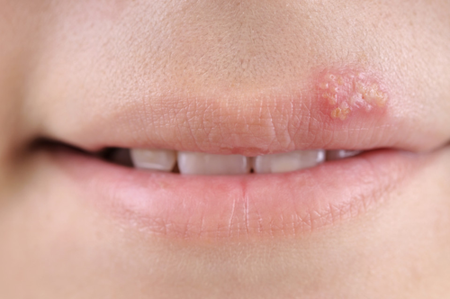
- treat cold sores (in adults and adolescents over 12 years old)
- prevent infection with CMV after organ transplants (in adults and adolescents over 12 years old)
- treat and prevent HSV infections of the eye
Before you take Valtrex
Don’t take Valtrex if you are allergic (hypersensitive) to valaciclovir or aciclovir or any of the other ingredients (listed in Section 6).
Don’t take Valtrex if this applies to you. If you are not sure, talk to your doctor or pharmacist before taking Valtrex.
Take special care with Valtrex
Check with your doctor or pharmacist before taking the oral pill Valtrex 250 mg, 500 mg, 1000 mg if:
- you have kidney problems
- you have liver problems
- you are over 65 years of age
- your immune system is weak
If you are not sure if the above apply to you, talk to your doctor or pharmacist before taking Valtrex 250 mg, 500 mg, 1000 mg film-coated tablets.
Prevent passing genital herpes on to others
If you are taking Valtrex to treat or prevent genital herpes, or you have had genital herpes in the past, you should still practice safe sex, including the use of condoms. This is important to prevent you passing the infection on to others. You should not have sex if you have genital sores or blisters.
Taking other medicines
Tell your doctor or pharmacist if you are taking or have recently taken any other medicines. This includes medicines obtained without a prescription, including herbal medicines.
Tell your doctor or pharmacist if you are taking any other medicines that affect the kidneys. These include: aminoglycosides, organoplatinum compounds, iodinated contrast media, methotrexate, pentamidine, foscarnet, ciclosporin, tacrolimus, cimetidine and probenecid.
Always tell your doctor or pharmacist about other medicines if you are taking Valtrex for treatment of shingles or after having an organ transplant.
Pregnancy and breast-feeding
Valtrex is not usually recommended for use during pregnancy. If you are pregnant, or think you could be, or if you are planning to become pregnant, don’t take Valtrex without checking with your doctor. Your doctor will weigh up the benefit to you against the risk to your baby of taking Valtrex while you’re pregnant or breastfeeding.
Driving or using machines
Valtrex can cause side effects that affect your ability to drive. -»Don’t drive or use machines unless you are sure you’re not affected.
How to take Valtrex oral tablets
Always take Valtrex exactly as your doctor has told you. You should check with your doctor or pharmacist if you are not sure.
The dose that you should take will depend on why your doctor has prescribed Valtrex for you. Your doctor will discuss this with you.
Treatment of shingles
The usual dose is 1000 mg (one 1000 mg tablet or two 500 mg tablets) three times a day.
You should take Valtrex for seven days.
Treatment of cold sores
The usual dose is 2000 mg (two 1000 mg tablets or four 500 mg tablets) twice a day.
The second dose should be taken 12 hours (no sooner than 6 hours) after the first dose
You should take Valtrex for one day (two doses) only.
Treatment of HSV infections of the skin and genital herpes
The usual dose is 500 mg (one 500 mg tablet or two 250 mg tablets} twice a day.
For the first infection you should take Valtrex for five days or for up to ten days if your doctor tells you to. For recurrent infection the duration of treatment is normally 3-5 days.
Helping to prevent HSV infections from returning after you have had them
The usual dose is one 500 mg tablet once a day.
Some people with frequent recurrences may benefit from taking one 250 mg tablet twice a day.
You should take Valtrex until your doctor tells you to stop.
To stop you being infected with CMV (Cytomegalovirus)
The usual dose is 2000 mg (two 1000 mg tablets or four 500 mg tablets) four times a day.
You should take each dose about 6 hours apart.
You will usually start taking Valtrex as soon as possible after your surgery.
You should take Valtrex for around 90 days after your surgery, until your doctor tells you to stop.
Your doctor may adjust the dose of Valtrex if:
- you are over 65 years of age
- you have a weak immune system
- you have kidney problems.
Talk to your doctor before taking Valtrex if any of the above apply.
Taking this medicine
Take this medicine by mouth.
Swallow the tablets whole with a drink of water.
Take Valtrex at the same time each day.
Take Valtrex according to instructions from your doctor or pharmacist.
People over 65 years of age or with kidney problems It is very important while you are taking Valtrex that you drink water regularly during the day. This will help to reduce side effects that can affect the kidney or nervous system. Your doctor will closely monitor you for signs of these. Nervous system side effects might include feeling confused or agitated, or feeling unusually sleepy or drowsy.
If you take more Valtrex than you should
Valtrex is not usually harmful, unless you take too much over several days. If you take too many tablets you may feel sick, vomit, or be confused, agitated or unusually sleepy. Talk to your doctor or pharmacist if you take too much Valtrex. Take the medicine pack with you.
If you forget to take Valtrex
If you forget to take Valtrex, take it as soon as you remember. However, if it is nearly time for your next dose, skip the missed dose.
Don’t take a double dose to make up for a forgotten dose.
Valaciclovir: Organs and Systems
Nervous system
Valaciclovir is a prodrug of aciclovir and can therefore cause similar effects, as two cases of nervous system effects have demonstrated.
- A 65-year-old man was given valaciclovir 1 g bd for 36 hours and had reduced concentration and was incoherent. All investigations were normal or negative. He improved rapidly on withdrawal of valaciclovir.
- A 44-year-old man was given valaciclovir 1 g tds for 5 days and developed a fever, disorientation, confusion, ataxia, dysarthria, and photophobia. All investigations were normal or negative. He was given antimicrobial drugs, including aciclovir, but his symptoms did not improve until the aciclovir was withdrawn.
Elderly patients and people with chronic renal insufficiency are most susceptible to the neurotoxic effects of aciclovir: confusion, hallucinations, dizziness, irritability, ataxia, tremor, myoclonus, and seizures. The symptoms usually occur within 3 days of the start of therapy and resolve within 5 days after withdrawal. Plasma aciclovir concentrations do not correlate with symptoms. Lumbar puncture and CT scans of the head are essentially unremarkable. The most common electroencephalographic abnormality is diffuse generalized slowing of brain wave activity.
Psychological, psychiatric
At high doses (8 g/day) hallucinations and confusion were a significant concern, but similar symptoms have also occurred at lower doses and in patients with renal insufficiency.
Ocular and auditory hallucinations have been reported in a 60-year-old female patient on CAPD.
A 58-year-old man with chronic renal insufficiency, who was hemodialysed twice a week, was treated with valaciclovir (1 g tds) for Herpes zoster. Two days later he became disoriented, dizzy, dysarthric, and experienced hallucinations. The serum aciclovir concentration was 21 µg/ml. Treatment was discontinued and he was treated with hemodialysis for 6 hours, resulting in marked clinical improvement. The next day his symptoms of dysarthria recurred, but immediately and completely resolved after a second hemodialysis.
Hematologic
In one study, high-dose valaciclovir was associated with an increased risk of a thrombotic microangiopathy-like syndrome, reported as thrombocytopenic purpura or hemolytic-uremic syndrome. This syndrome occurred in 14 of 523 patients who received valaciclovir and in only four of 704 patients who received aciclovir after a median of 54 (range weeks of treatment.
The precise relation to valaciclovir remains unclear, since eight of 14 patients who were treated with valaciclovir had stopped treatment at least 1 week before the onset of the syndrome. In addition, all patients with thrombotic microangiopathy-like syndromes had taken multiple concomitant medications, and most had other intercurrent illnesses, which could have explained the hematological and renal abnormalities. The authors concluded that additional data are required to understand the role of valaciclovir and other medications for thrombotic microangiopathy-like syndromes, which are recognized with increasing frequency in patients with advanced HIV disease.
Gastrointestinal
Nausea, vomiting, and abdominal pain were commonly reported in human volunteers, but only diarrhea was significantly associated with exposure.
Urinary tract
Aciclovir is excreted renally. High plasma concentrations of aciclovir can lead to precipitation in renal tubules, causing impaired renal function, which is generally reversible. Since oral valaciclovir can result in plasma aciclovir concentrations comparable to those attained with intravenous dosing, reversible impairment of renal function can also occur after prolonged use of high-dose valaciclovir. Indeed, in a study of high-dose valaciclovir for prevention of cytomegalovirus disease in HIV-infected people, there was an association between treatment with valaciclovir and moderate nephrotoxicity (serum creatinine more than 1.5 times the upper limit of normal; estimated creatinine clearance under 50 ml/ minute).
Valaciclovir: Side Effects
Valaciclovir is the L-valyl ester of aciclovir. After oral administration, it is rapidly and extensively converted to aciclovir by first-pass metabolism, resulting in plasma aciclovir concentrations previously only attainable with intravenous administration. Like aciclovir, valaciclovir is generally well tolerated. Compared with oral aciclovir, the systemic availability of aciclovir from oral valaciclovir is markedly improved.
Valaciclovir is highly active against Herpes simplex and Herpes zoster. It is also effective in suppressing recurrent episodes of genital herpes. Prophylactic administration of high doses of valaciclovir to prevent CMV disease was effective in patients with AIDS and in liver transplant recipients.
Observational studies
In a double-blind comparison of two regimens of valaciclovir 500 mg bd for recurrent genital herpes, a 5-day course and a 3-day course, there were no significant differences in therapeutic outcome or adverse events between the two regimens. The most common adverse events were headache (10%), nausea (4%), diarrhea (3%), and fatigue (1.5%).
Comparative studies
The effects of aciclovir and valaciclovir for anogenital herpes have been studied in HIV-infected individuals in two controlled trials. In the first study, 1062 patients with CD4+ counts over 100 x 106/1 received valaciclovir or aciclovir for 1 year and were assessed monthly. In the second study, 467 patients were treated episodically for at least 5 days with valaciclovir or aciclovir and were assessed daily. Valaciclovir was as effective as aciclovir for suppression and episodic treatment of herpesvirus infections. Hazard ratios for the time to recurrence with valaciclovir 500 mg bd and 1000 mg od compared with aciclovir were 0.73 (95% CI = 0.50) and 1.31 (0.94). Valaciclovir 1000 mg bd and aciclovir had similar effects on the duration of infective episodes (HR — 0.92; CI = 0.75). The most common adverse events, which occurred at similar rates with all regimens, were diarrhea, headache, infections, rashes, nausea, rhinitis, pharyngitis, abdominal pain, fever, depression, and cough.
Placebo-controlled studies
In large, placebo-controlled comparisons of the efficacy of valaciclovir and aciclovir in treating or suppressing recurrent genital Herpes simplex infections in immuno-competent people, dosages up to 2 g/day were well tolerated, with safety profiles comparable to aciclovir. In a comparison of high-dose valaciclovir (8 g/day) with two doses of aciclovir (0.8 and 3.2 g/day) for prophylaxis of cytomegalovirus disease in patients with advanced human immunodeficiency virus infection, intention-to-treat analysis showed a trend toward earlier mortality in those who received valaciclovir. In those who actually received valaciclovir, survival was significantly shorter. In view of the unexplained trend toward earlier mortality, as well as higher frequencies of renal toxicity (see the section on Urinary tract) and premature treatment discontinuation, the authors concluded that the dose of valaciclovir was too high and that better tolerated doses, which maintain a protective effect on cytomegalovirus disease, need to be identified.
Second-Generation Effects
Fetotoxicity
In a phase I trial, valaciclovir administered in the third trimester of pregnancy was well tolerated.
Susceptibility Factors
Renal disease
Adverse effects of valaciclovir, the L-valyl ester of aciclovir, can be associated with increased drug concentrations when the dose is not adjusted for reduced renal function. For example, aseptic meningitis has been associated with valaciclovir in a patient with renal insufficiency.
An 88-year-old man with renal insufficiency took valaciclovir 1000 mg tds. After the first dose, he became disoriented and incontinent. Valaciclovir was withdrawn, but the symptoms continued and progressed to drowsiness and nuchal rigidity. After an extensive work-up, aseptic meningitis was diagnosed.
Given the patient’s age and renal dysfunction, it is likely that excessive valaciclovir accumulation was responsible for this presentation.
Drug-Drug Interactions
Cimetidine
In an open, single-dose study of the effects of probenecid and cimetidine on the pharmacokinetics of valaciclovir and its metabolite aciclovir in 12 healthy men, valaciclovir 1 g, valaciclovir plus probenecid 1 g, valaciclovir plus cimetidine 800 mg, and valaciclovir with a combination of probenecid and cimetidine were studied. At three subsequent administrations, drug regimens were alternated among groups so that each group received each regimen. Probenecid and cimetidine respectively increased the mean Cmax of valaciclovir by 23 and 53% and its AUC by 22 and 73%. Probenecid and cimetidine also respectively increased the mean aciclovir Cmax by 22 and 8% and its AUC by 48 and 27%. The combination had a greater effect than either drug alone. Neither cimetidine nor probenecid affected the absorption of valaciclovir.
Probenecid
In an open, single-dose study of the effects of probenecid and cimetidine on the pharmacokinetics of valaciclovir and its metabolite aciclovir in 12 healthy men, valaciclovir 1 g, valaciclovir plus probenecid 1 g, valaciclovir plus cimetidine 800 mg, and valaciclovir with a combination of probenecid and cimetidine were studied. At three subsequent administrations, drug regimens were alternated among groups so that each group received each regimen. Probenecid and cimetidine respectively increased the mean Cmax of valaciclovir by 23 and 53% and its AUC by 22 and 73%. Probenecid and cimetidine also respectively increased the mean aciclovir Cmax by 22 and 8% and its AUC by 48 and 27%. The combination had a greater effect than either drug alone. Neither cimetidine nor probenecid affected the absorption of valaciclovir.
How to store
Before purchase Valaciclovir, you must read how to store
Keep out of the reach and sight of children.
Do not use Valtrex after the expiry date which is stated on the carton. The expiry date (Exp.) refers to the last day of that month.
Store below 30°C.
Medicines should not be disposed of via wastewater or household waste. Ask your pharmacist how to dispose of medicines no longer required. This will help protect the environment.
Further information about Valtrex
What Valtrex contains
The active substance is valaciclovir. Each tablet contains 250 mg or 500 mg of valaciclovir (as valaciclovir hydrochloride).
The other ingredients are:
Tablet core
- Microcrystalline cellulose Crospovidone Povidone
- Magnesium stearate Colloidal silicon dioxide
Film coat
- Hypromellose
- Titanium dioxide
- Macrogol
- Polysorbate 80 (500 mg tablets only)
- Blue printing ink FT203 containing brilliant blue (E133) (250 mg tablets only)
- Carnauba wax
What Valtrex tablets look like and contents of the pack
Valtrex tablets are contained in polyvinyl chloride/aluminium foil blister packs.
Valtrex Tablets 250 mg are supplied to you in cartons containing 60 film-coated tablets. They are white and marked with “GX CE7” on one side.
Valtrex Tablets 500 mg are supplied in cartons containing 10,30, 42, 90 or 112 film-coated tablets. They are white and marked with “GX CF1” on one side.
| Dosage forms of Valaciclovir: | |||
|---|---|---|---|
| Apo-Valacyclovir (Caplet) 500 mg Tablet | Pms-Valacyclovir (Caplet) 500 mg Tablet | Valtrex (Caplet) 500 mg Tablet | Valacyclovir HCl 500 mg tablet |
| Valtrex 500 mg tablet | Valacyclovir HCl 1 gm tablet | Valtrex 1 gm caplet | Valtrex 1 gm tablet |
Synonyms of Valaciclovir:
Valaciclovir, Valaciclovir Hcl, Valaciclovir Hydrochloride, Valacyclover Hydrochloric, Valacyclover Hydrochloride, Valacyclovir, Valacyclovir Hydrochloride
How can i get Valaciclovir online over the counter?
You can buy Valaciclovir OTC in online drugstore with low cost.
Therapeutic classes of Valaciclovir:
Antiviral Agents, Prodrugs
Delivery
Australia, Canada, Mexico, New Zealand, USA, Europe [Belgium, France, Norway, Holland, Ireland, Spain, Switzerland, Great Britain (UK), Italy] and etc.


 (11 votes, average: 4.18 out of 5)
(11 votes, average: 4.18 out of 5)