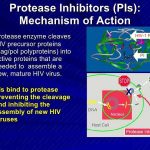
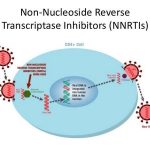
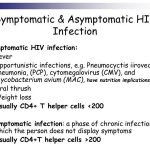
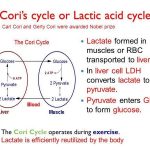
While further study is needed, data are accumulating regarding pharmacokinetic interactions among the various antiretroviral agents, especially those involving the HIV protease inhibitors and NNRTIs, and the need for dosage adjustments as a result of these interactions. While some pharmacokinetic interactions between antiretroviral agents can be used for therapeutic advantage (e.g., use of low-dose ritonavir to boost plasma concentrations of some other HIV protease inhibitors), other interactions can result in suboptimal drug concentrations and reduced therapeutic effects and should be avoided. The pharmacokinetic interaction between ritonavir and other HIV protease inhibitors is now used for therapeutic advantage in various antiretroviral regimens.