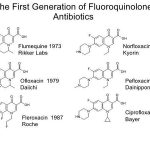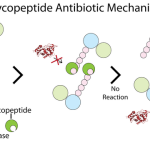
Many bacteria have been shown to cause community-acquired pneumonia, but researchers and clinicians identify several bacterial species as the most common causes of the disease. However, epidemiological studies investigating the etiology of the disease show that in the majority of cases, pathogen identification is not readily attainable. Pathogens are classified as typical or atypical based on the spectrum of symptoms associated with them (described in the preceding section). In most studies, five pathogens (discussed in the following sections) have been found to account for approximately 90% of all community-acquired pneumonia.