Essentials of Diagnosis
- Positive serum immunoglobulin G usually indicates active infection.
- Serology is generally not helpful for documentation of cure.
- Urea breath test and stool antigen test are useful to document cure.
- Acid suppression therapy decreases the sensitivity of the urea breath test.
- Culture and susceptibility testing may be useful in refractory cases.
General Considerations
Pathologists have noted spiral bacteria in biopsies and autopsy specimens of gastric mucosa for over 100 years. Their significance was alternately debated and ignored until 1982, when Barry Marshall and Robin Warren cultivated the organism for the first time and suggested that it might be a cause of chronic gastritis and peptic ulcer disease. Although initially called Campylobacter pylori, subsequent taxonomic studies showed that the bacterium was not a true Campylobacter species, and it was renamed Helicobacter pylori. Despite initial skepticism, Marshall and Warren’s early proposal that H pylori caused gastritis and peptic ulcer disease proved correct.
Furthermore, there is now overwhelming evidence that H pylori is linked to adenocarcinoma and non-Hodgkin’s lymphoma of the stomach.
The clinical significance of this organism has been emphasized by a National Institutes of Health consensus panel that recommended antibiotic therapy for the large majority of peptic ulcer patients who are infected with H pylori and by the classification of H pylori as a carcinogen by the World Health Organization.
Epidemiology
Prevalence of infection
H pylori is one of the most prevalent bacterial disease agents of humans and has been isolated from human stomachs in all parts of the world. In developing countries the prevalence is 70-90%, and most persons acquire H pylori infection before the age of 10 years. In the United States and other developed countries, approximately one-third of the population is infected. The prevalence of infection is greater among African-Americans and Hispanics than non-Hispanic whites, which seems to be explained only partially by socioeconomic factors. Prevalence also increases with age, from ~ 10% in children to ~ 50% by 50 years of age. In part, this is because, with each year of life, there is an incremental increase in chances of acquiring H pylori infection. However, probably more important is the “cohort effect”; because infection is typically acquired in childhood and the overall prevalence of H pylori in developed countries is declining, individuals born 50 years ago (when H pylori was more common) are found more often to be infected than persons born more recently. Once acquired, infection is persistent, usually for the lifetime of the patient.
Transmission
There are no well-documented environmental reservoirs of H pylori. Only humans and some nonhuman primates are infected. While it is known that infection is transmitted from person to person, the exact mechanism is unclear. H pylori cannot generally be cultivated from stools of infected patients, although some evidence suggests that cultures may be positive if patients have diarrhea. Recent cultivation of H pylori from vomitus raises the intriguing possibility that transmission occurs during episodes of gastroenteritis by the gastro-oral route.
Prevalence of disease
Although all persons infected with H pylori will have histologic evidence of gastritis, only ~ 15-20% will have clinical disease at some point in their lifetime that is associated with H pylori infection (Table 1). Thus, although it clearly is a pathogen (recall that only ~ 20% of patients infected with Mycobacterium tuberculosis will have disease at some point in their lives), most people infected with H pylori will never have clinical sequelae of infection.
Microbiology
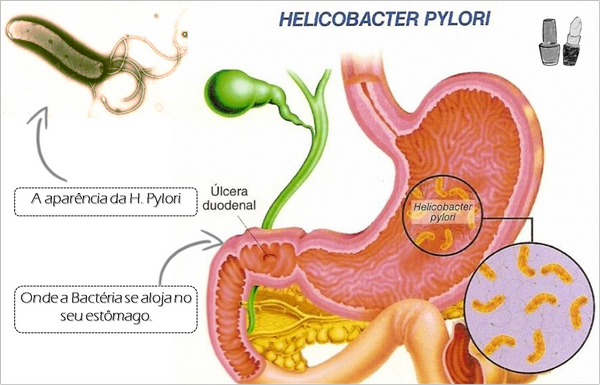
H pylori is a microaerophilic gram-negative rod that has a gently curved or S-shaped morphology that resembles Campylobacter spp. The organism displays a unique “corkscrew” motility produced by its four to eight unipolar flagella.
Optimal growth occurs at 37 °C under microaerobic conditions on brucella agar or other rich medium supplemented with blood or serum. Since cultivation of H pylori from gastric biopsy requires 4-7 days, agar plates are typically supplemented with antibiotics (trimethoprim, vancomycin, polymyxin-B, and amphotericin B) to suppress overgrowth by fungi and other bacteria. H pylori is identified by the formation of pinpoint translucent colonies, its gram-negative staining characteristics, and positive tests for oxidase, catalase, and urease.
Urease is produced in abundance by H pylori and is the basis for two tests commonly used to diagnose H pylori infection. Analysis of the complete H pylori genome reveals a large number of genes that are predicted to encode ion pumps, but few genes that encode regulatory factors; these findings are consistent with the notion of an organism that has adapted to a highly acidic environment. “Helicobacter heilmannii” is a related uncultivated bacterium that is uncommon but appears also to be associated with gastritis, peptic ulcer disease, and gastric malignancy. Several other species of Helicobacter can occasionally infect the large intestines and perhaps the hepatobiliary system, and they are sometimes associated with diarrheal disease.
Pathogenesis
Histopathology
All persons infected with H pylori have a chronic gastritis characterized by mononuclear inflammatory cells as well as an associated neutrophilic infiltration. Both the organism and the gastritis tend to be located preferentially in the gastric antrum. The role of H pylori in causing this chronic gastritis, previously thought to be a natural result of aging, has been clearly demonstrated by the finding that eradication of H pylori eliminates the gastritis and by two experimental self-inoculation studies in which physicians have voluntarily ingested H pylori. The organism can be found predominantly in the mucus gel layer, although a minority of bacteria will be adherent to the gastric epithelium. H pylori exhibits a profound tissue tropism, and it attaches only to gastric epithelium in the stomach or to epithelium in the proximal duodenum that has undergone gastric metaplasia.
Mechanisms of tissue injury
Polar flagella and a spiral morphology permit H pylori to move within gastric mucus and, on occasion, attach to gastric epithelium. Because the majority of bacterial cells are not attached to the epithelium, soluble factors are thought to play a role in generating the inflammatory response. About 60% of strains isolated from patients in the United States produce a vacuolating cytotoxin called VacA, which induces acidic vacuoles in the cytoplasm of eukaryotic cells.
Although the exact role of VacA in disease is unknown, patients infected with strains that produce VacA are more likely to develop peptic ulcer disease and gastric adenocarcinoma than patients infected with strains that do not produce VacA. Interestingly, all H pylori strains have the gene for VacA, but cytotoxin activity is, for the most part, found only in those strains that also contain a 40-kilobase pathogenicity island called CagA.
These strains also have the ability to induce interleukin-8, which serves to recruit and activate neutrophils. Like all gram-negative bacteria, H pylori produces lipopolysaccharide, which can disrupt gastric mucus. Interestingly, the H pylori lipopolysaccharide has a relatively low proinflammatory activity compared with that from enteric gram-negative bacteria such as Escherichia coli. This probably reflects its ability to coexist with the host over decades, usually causing little overt disease. The histologic gastritis may also reflect autoimmune mechanisms, because portions of the H pylori lipopolysaccharide side chains mimic host Lewis antigens expressed on gastric epithelium. H pylori also induces gastric mucosal injury by provoking apoptosis.
H pylori urease hydrolyzes urea into ammonia and carbon dioxide, which is then converted to bicarbonate. Urease is essential for H pylori colonization probably in part because the bicarbonate protects the bacterium from the effects of gastric acid. Urease also has toxic effects on gastric epithelial cells, so it functions both as colonization and virulence factor.
Altered gastric homeostasis
H pylori infection induces the expression of gastrin, an acid-stimulating peptide, and suppresses expression of the acid-inhibiting somatostatin. However, gastric acid production is not always increased in patients infected with H pylori. During the first few weeks to months of infection, there is thought to be a transient hypochlorhydria. After this stage, there may be increased or eventually decreased gastric acid, depending on a variety of factors. In general, gastric acid is increased in patients who develop duodenal ulcers but decreased in patients who develop gastric cancer.
Clinical Findings
Acute Infection
Limited human inoculation studies suggest that acute H pylori infection may be associated with nausea, vomiting, upper abdominal pain, and bloating. It seems likely that some patients diagnosed with “gastroenteritis” have acute H pylori infection, particularly children, in whom most infections occur. Symptoms last several days and resolve spontaneously. There are no currently available methods for detection of acute H pylori infection, so the diagnosis is virtually never made. Because the acute illness is transient and most patients will not develop clinical sequelae of infection, there is little reason to look for H pylori infection in patients with a syndrome of gastroenteritis. Like many infectious diseases, H pylori acquisition may be asymptomatic.
Chronic Active Gastritis
Most if not all patients who acquire H pylori infection will remain persistently colonized and will develop chronic active gastritis. This is a pathologic and not a clinical diagnosis, which should not be confused with erosive or other forms of gastritis that can be diagnosed by gross visual examination with an endoscope.
Peptic Ulcer Disease
Approximately 15-20% of H pylori-infected persons will develop peptic ulcer disease at some point in their lifetimes. If one excludes ulcers due to nonsteroidal anti-inflammatory drugs (NSAIDs), > 80% of peptic ulcers can be attributed to H pylori infection. This conclusion is based on the observation that H pylori-associated ulcers treated with acid suppression alone will commonly recur when therapy is stopped after initial healing, while those treated with antibiotics that eradicate H pylori recur much less often. Since ulcers related to NSAIDs are more often gastric than duodenal, the association between H pylori infection and duodenal ulcer is stronger than that with gastric ulcer. In the absence of NSAIDs or other conditions such as Zollinger-Ellison syndrome or Crohn’s disease, physicians should presume that ulcers in patients infected with H pylori are caused by the infection.
Gastric Malignancy
Although uncommon in the United States and other developed countries, gastric adenocarcinoma is the second leading cause of cancer worldwide and the second leading cause of cancer death. Persons infected with H pylori have approximately a sixfold increased risk of developing gastric cancer compared with those that are uninfected. Although the incidence of gastric cancer in the United States is declining, it remains relatively common among Hispanics, African-Americans, and Asians. These populations, like those in developing countries, have a higher prevalence of H pylori infection and are typically infected at a younger age than non-Hispanic whites. Overall, < 1% of infected persons in developed countries will develop gastric cancer, but, in developing countries, particularly in Asia, the risk may be as high as 10%.
Gastric cancer and duodenal ulcer disease are almost mutually exclusive outcomes of H pylori infection. Persons in developed countries are more likely to develop duodenal ulcer, while those in developing countries more commonly develop gastric cancer. It is thought that the difference lies in whether infection produces high gastric acid, which may lead to duodenal ulcer, or low gastric acid with atrophic gastritis, which is the histologic precursor to gastric adenocarcinoma. The factors that determine whether one progresses down one path or the other are largely unknown.
Gastric mucosa-associated lymphoid tissue (MALT) lymphoma is a rare disease that is strongly associated with H pylori infection. If identified early, the tumor can be cured with effective H pylori therapy in > 75% of patients. Therefore, antibiotic therapy is considered the standard of care for treatment of patients with H pylori infection and gastric MALT lymphoma.
Nonulcer Dyspepsia
Nonulcer dyspepsia is a poorly defined entity whose etiology is not well understood. Several large randomized clinical trials have now evaluated the effects of antibiotics for H pylori on nonulcer dyspepsia. In general, antibiotic therapy produces symptomatic improvement in 20-25% of patients; however, these results are similar in placebo-treated controls.
Other Diseases and Syndromes
There are multiple reports of associations between H pylori infection and cardiovascular disease, as well as several rheumatologic diseases. Since H pylori infection is so common, a causal relationship is difficult to demonstrate, and none has yet been established.
Diagnosis
Several methods for diagnosis of H pylori are available, both noninvasive and endoscopic (Table 2).
Serology
Serum enzyme-linked immunosorbent assay for immunoglobulin G is sensitive and specific for detection of H pylori infection. Presuming that the patient has not been recently treated for H pylori, in which case an antibody test may be falsely positive because seroreversion has not yet occurred, a positive serum immunoglobulin G for H pylori indicates that there is active infection in the gastric mucosa. H pylori serum immunoglobulin M measurements are reported by some commercial laboratories, but the methods have not been well standardized and they are currently of no clinical value. Assays based on whole blood are generally less accurate and offer no significant advantages over those based on serum.
Urea Breath Test
Two urea breath tests have been approved by the U.S. Food and Drug Administration. The patient ingests either [14C]-urea or [13C]-urea. If H pylori is present in the stomach, these substrates are hydrolyzed and either [14C]- or [13C]-labeled CO2 can be measured in a sample of expired air. Both tests are highly sensitive and specific for active H pylori infection. 13C is a naturally occurring radioisotope of carbon and involves no ionizing radiation exposure to the patient. The radiation exposure from a [14C]-urea breath test is negligible, amounting to approximately the excess radiation dose accumulated by a transcontinental airline flight. Patients should avoid taking H2 receptor antagonists or proton pump inhibitors for 1-2 weeks before a urea breath test, because these agents increase the likelihood of a false-negative test.
Endoscopy
Histologic examination of an endoscopic biopsy is a sensitive test for H pylori when performed by an experienced pathologist, and the specificity is nearly 100%. Organisms are most easily seen with silver stains such as Warthin-Starry or Genta, but routine hematoxylin and eosin or Giemsa stains are usually sufficient. Multiple biopsies should be obtained because the infection is not uniformly distributed. A rapid urease test may also be performed on gastric biopsies by placing the sample in a vial containing urea and a pH-sensitive dye, which will change color in the presence of bacterial urease activity.
This test is inexpensive and accurate, and the results may be available in as soon as a few hours. Cultivation of H pylori from gastric biopsies is not commonly performed because it requires special media, and laboratories are often not experienced with growing this fastidious organism.
However, cultivation offers the unique advantage over other techniques that the isolate is available for antibiotic susceptibility testing. This may be particularly important in patients who have failed initial attempts at therapy. If a local laboratory cannot cultivate H pylori, samples can be placed directly in a sterile tube, frozen (preferably at -70 °C, but -20 °C will usually suffice), and shipped on dry ice to a reference laboratory.
Stool Antigen Test
A recently developed stool antigen test is approved by the U.S. Food and Drug Administration for evaluation of H pylori infection. It is > 90% sensitive and specific for detection of chronic infection, as well as for confirming eradication of H pylori when performed 4 weeks after completion of antibiotic therapy.
Treatment
Indications for Antibiotic Therapy
All patients with peptic ulcer disease and H pylori infection should be treated with antibiotics, unless an alternative cause such as NSAIDs can be clearly demonstrated. This includes patients newly presenting with ulcer disease as well as those with a well-documented history of ulcer. Often these latter patients will be intermittently taking H2 blockers or proton pump inhibitors, which can be stopped after effective antibiotic therapy.
Patients with MALT lymphoma should also receive antibiotic treatment for H pylori. Cost-benefit analyses have suggested that it may be economical to treat empirically patients who have dyspepsia and H pylori infection. However, the now documented failure of H pylori therapy to ameliorate the symptoms of nonulcer dyspepsia, together with growing problems with antibiotic resistance, renders this strategy less attractive.
Furthermore, controversial data suggest that eradication of H pylori may be associated with an increased incidence of gastroesophageal reflux disease (GERD) and carcinoma of the gastroesophageal junction. Preventive antibiotic therapy may also be considered in a patient with H pylori infection and a family history of gastric adenocarcinoma.
Because long-term therapy with acid suppression in H pylori-infected patients with GERD has sometimes been associated with increased risk of atrophic gastritis, some have suggested that these patients receive therapy for H pylori to prevent gastric cancer. This remains controversial, particularly in light of the evidence that H pylori eradication may exacerbate symptoms of GERD. H pylori screening and treatment in patients with no symptoms and no risk factors for gastric cancer are not indicated based on current data.
Antibiotic Regimens
Several general guidelines are helpful to identify a proper treatment regimen. First, results for all proton pump inhibitors studied to date are essentially equivalent, so the choice should be guided primarily by cost. Second, related antibiotics in a class should not be substituted for those that are recommended.
For example, amoxicillin and tetracycline should not be replaced with ampicillin and doxycycline, respectively, nor should any macrolide be used other than clarithromycin, until further studies are done. Third, all effective regimens include at least two antibiotics in addition to acid suppression.
Finally, antibiotic therapy should be continued for a minimum of 1 week, although 2 weeks is preferable. In ulcer disease the acid suppression therapy should be administered for 2 additional weeks.
Many treatment regimens are available that yield cure rates of = 80-90% and differ primarily in cost, convenience, and side effects rather than in efficacy (Box 1). The initial regimen developed for H pylori treatment was a combination of an H2 blocker twice daily, tetracycline four times daily, bismuth subsalicylate four times daily, and metronidazole three times daily. Amoxicillin can be substituted for tetracycline, although there may be some marginal loss of efficacy.
A proton pump inhibitor is now commonly substituted for the H2 blocker. An effective alternative is a proton pump inhibitor or ranitidine bismuth citrate (a combination of ranitidine and bismuth) each given twice daily together with two of the following: amoxicillin, clarithromycin, or metronidazole, each given twice daily. The latter approach is significantly more expensive than the bismuth-containing regimen, but twice-daily dosing may promote adherence.
Follow-up Testing
In the past, follow-up testing has not been routinely recommended, in part because noninvasive methods were not available. Serology is generally not useful for follow-up because a prolonged period is required for seroreversion after effective therapy. Currently, both the urea breath test and the stool antigen test are noninvasive methods that accurately detect H pylori eradication when performed = 4 weeks after completion of antibiotic therapy.
Patients with gastric ulcer, particularly those in whom the clinical history raises concern about gastric cancer, and patients with MALT lymphoma, should undergo follow-up endoscopy after completion of therapy. Patients who are still infected with H pylori after therapy are likely never to have cleared the organism or are likely to have recrudescent infection rather than reinfection. The reinfection rate in adults after successful therapy documented = 4 weeks after treatment is < 1% per year.
Antibiotic Resistance
Antibiotic resistance to metronidazole is present in ~ 30% of H pylori strains in the United States, but can be as high as 90% in some populations, depending on the frequency of metronidazole use. A clear relationship between in vitro resistance and clinical efficacy has not been consistently demonstrated; this probably reflects heterogenous mechanisms of resistance. Clarithromycin resistance occurs in 5-15% of strains.
It is associated with mutations in the 23S rRNA gene and results in marked loss of efficacy. Because clarithromycin and metronidazole are the two agents for which resistance most commonly develops, it may be prudent not to use them together as initial therapy, to avoid development of resistance to both agents. Resistance to amoxicillin, bismuth, and tetracycline in H pylori strains is rare.
Therapy in Treatment Failures
The patient in whom the initial regimen fails to eradicate adherent H pylori should be treated with an alternative combination (Table 55-3). If metronidazole or clarithromycin was part of the original treatment, it is reasonable to presume that resistance has developed and to substitute clarithromycin or metronidazole, respectively, or to use amoxicillin. Resistance to metronidazole may sometimes be overcome by increasing the dose to 500 mg three times daily or even four times daily if tolerated.
One may also consider sending a gastric biopsy for culture and susceptibility testing to a research lab if such testing is not locally available. Susceptibility testing should be performed against clarithromycin, metronidazole, amoxicillin, and tetracycline by the agar dilution method. This method has replaced the epsilometer agar diffusion gradient (E-Test, AB Biodisk) for H pylori antibiotic resistance testing.
Treatment of H pylori Infection in Children
H pylori infection is uncommon among children in the United States and other developed countries, as are peptic ulcer disease and gastric malignancy. Although the same agents that are used to treat H pylori in adults appear to be effective in children, there are no standard guidelines on when or how children should be treated. H pylori therapy in children with gastrointestinal symptoms should not be undertaken without consultation from a pediatric gastroenterologist.
Prevention & Control
There are no available guidelines regarding prevention of infection. However, persons infected with H pylori who have diarrhea or vomiting should probably be considered contagious. Oral and parenteral vaccines, primarily based on H pylori urease, have been studied extensively in animal models, although none has proven effective at providing sterilizing immunity. Therapeutic-vaccination studies in animals and preliminary studies in humans are also ongoing.




