Essentials of Diagnosis
- Demonstration of the acid-fast bacillus.
- Infections more common in immunocompromised hosts.
- Infections mainly pulmonary or soft tissue.
General Considerations
The increasingly relative importance of the atypical mycobacteria, many of which are ubiquitous in the environment, was recognized with the decline in tuberculous disease. Generally, atypical mycobacteria are unusual causes of disease in patients who are immunocompetent but can in immunocompromised hosts such as AIDS and cancer patients. Most infections caused by atypical mycobacteria are skin and soft tissue abscesses, sometimes following pulmonary infection or implantation of prosthetic devices. There have been a few reports of epidemics of iatrogenic infection with atypical mycobacteria, associated with injection of contaminated materials.
Epidemiology
Atypical mycobacteria may account for 35% of isolations of potentially pathogenic mycobacteria in the United States; the rate of infection from atypical mycobacteria, however, ranges from 0.5% to 30% of all infections caused by mycobacteria. An estimated 3 million of the 6 million cases of leprosy worldwide remain untreated.
Microbiology
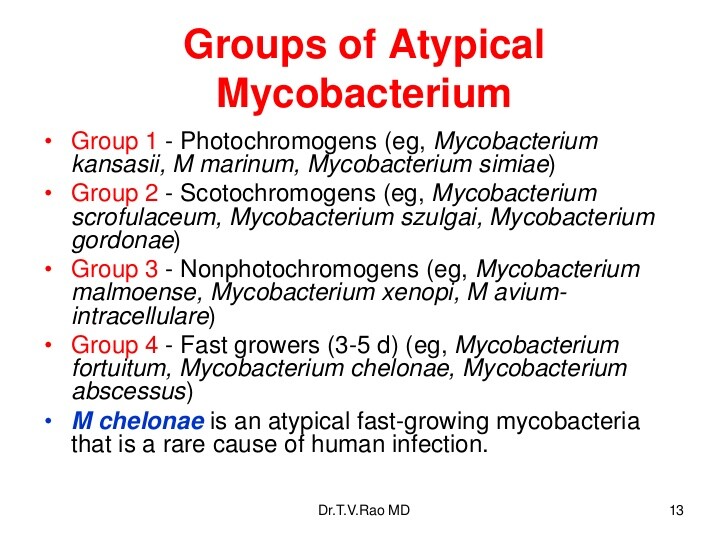
The atypical mycobacteria are classified into four groups according to their growth characteristics, as well as pigment production in culture. Runyon class I includes organisms such as Mycobacterium kansasii, which are characterized by pigment production upon exposure to light (photochromogens). Runyon class II, including Mycobacterium scrofulaceum, are scotochromogens, which produce pigment in the light or in the dark. Runyon class III includes nonpigment producers (nonchromogens) such as Mycobacterium avium complex (MAC). Runyon class IV organisms, such as Mycobacterium abscessus, are characterized by rapid growth in culture, on the order of 2-30 days, as compared with the other mycobacteria, which often take 6-8 weeks to cultivate.
Pathogenesis
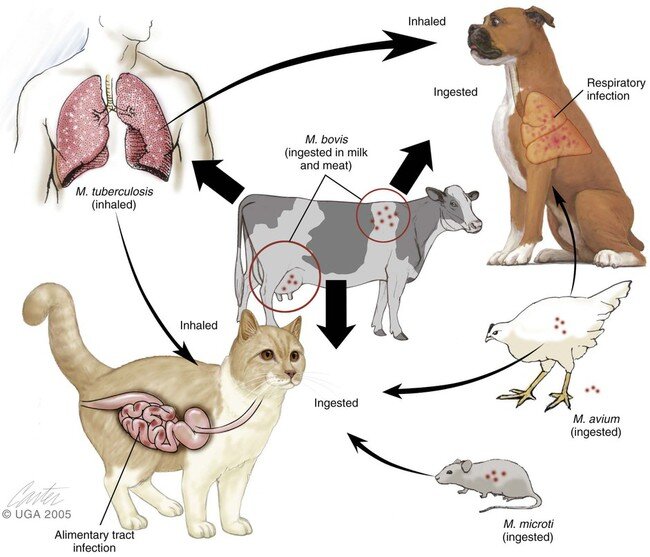
Sources of the atypical mycobacteria include soil, water, and domestic and wild animals (see Table 1 for habitats of common medically important mycobacteria). Most infections are acquired through aspiration or inoculation of atypical mycobacteria from the environment. Incubation periods are very long, averaging 5 years. Affected patients are of all age groups, but the peak age of onset is in young adults; children are rarely affected.
Pulmonary disease often results from inhalation of organisms, whereas direct inoculation of the organism or a foreign body contaminated with the organism results in soft-tissue disease. Ingestion of organisms can result in gastrointestinal involvement. Person-to-person transmission does not usually occur, with the exception of Mycobacterium leprae, which is transmitted by nasal droplets, although there is some evidence that transmission may occur by soil. Disseminated disease does not usually occur except in immunocompromised hosts.
Clinical Syndromes
Mycobacterium Avium Complex (Disseminated & Pulmonary Disease)
Mycobacterium Leprae (Leprosy)
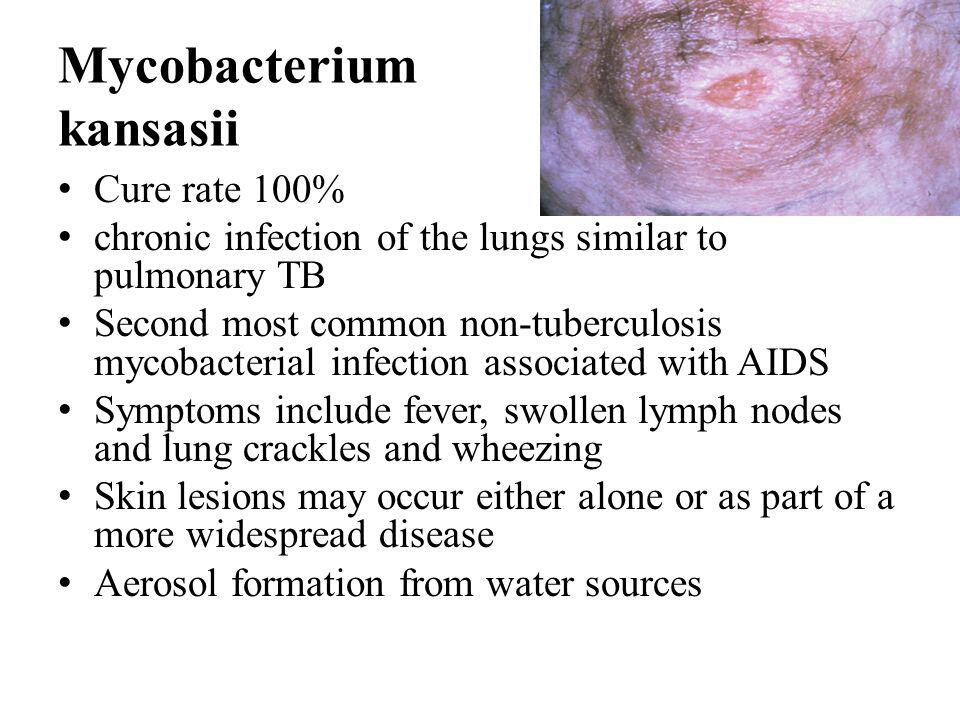
Mycobacterium Kansasii (Chronic Pulmonary Infection)
M kansasii is the most common nontuberculous mycobacterium after MAC to be isolated in tertiary care centers. M kansasii is a pathogen worldwide, and in the United States the incidence is highest in the Midwest and Southwest. It commonly affects individuals in certain occupations, such as miners, welders, and sandblasters. Patients with underlying pulmonary pathology can be infected also. However, M kansasii rarely causes disease in children. Children who are affected tend to be immunocompetent and without an underlying pulmonary disease.
Clinical Findings
The most common clinical syndrome caused by M kansasii is a chronic pulmonary infection that resembles pulmonary tuberculosis.
Signs and Symptoms
Symptoms of infection are nonspecific and can include cough, fatigue, and shortness of breath. Uncommonly, M kansasii can cause local lymphadenitis, a skin infection that resembles sporotrichosis. With disseminated infections, skin lesions can be present, such as erythema nodosum, erythema multiforme, and induration.
In AIDS patients, M kansasii is the second most common infection caused by atypical mycobacterium. It tends to produce a disseminated disease similar to MAC. M kansasii-disseminated disease was present at the time of the index AIDS diagnosis in 0.2% of patients. M kansasii can also produce isolated oral ulcers, osteomyelitis, soft tissue infections, tenosynovitis, and arthritis. It may also produce necrotizing pulmonary nodules.
Laboratory Findings
Nonspecific.
Imaging
On chest x-ray, M kansasii is difficult to distinguish from M tuberculosis. In children, mediastinal and hilar lymph nodes are commonly enlarged.
Differential Diagnosis
Atypical mycobacterium should be suspected if a patient does not respond to conventional therapy for M tuberculosis.
Mycobacterium Scrofulaceum (Lymphadenitis)
M scrofulaceum is a ubiquitous scotochromogenic mycobacterium that commonly exists in soil and water and contaminates reagents and foodstuffs. It readily colonizes respiratory secretions of healthy children and adults. The most common clinically evident disease in humans is lymphadenitis, which is commonly seen in children < 12 years old.
Clinical Findings
Signs and Symptoms
The clinical presentation is a single node or cluster of nodes in the submandibular area. The nodes slowly enlarge over weeks. The patient experiences very few local or systemic symptoms except for mild lymph node tenderness on palpation. Over weeks to months, if left untreated, the infection comes to the surface, ruptures, and forms a draining sinus, which then calcifies.
Laboratory Findings
Nonspecific.
Mycobacterium Bovis (Tuberculosis in Animals)
M bovis is considered to be part of the M tuberculosis complex. It is a nonchromogen and generally takes 21-40 days to isolate in culture. Its natural reservoir is humans and cattle, and it generally causes tuberculosis in cattle, goats, cats, dogs, primates, and other wildlife. It is rarely a cause of tuberculosis in humans, although there are reports of it causing 3% of the tuberculosis cases in certain places such as San Diego, California.
An attenuated strain of M bovis is used for bacille Calmette-Guerin (BCG) vaccine. Transmission of the organism occurs by inhalation of aerosol or by direct inoculation. Transmission by ingestion of contaminated milk from infected cows did occur prior to routine pasteurization. Clinical syndromes associated with M bovis include soft tissue infection. Soft tissue infection has been reported after accidental self-inoculation with BCG by a health care worker.
Clinical Findings
M bovis will cause pulmonary infection indistinguishable from that caused by M tuberculosis. Bacteremia, mycotic aortic aneurysm, vertebral osteomyelitis, and granulomatous hepatitis have been reported as complications of bladder instillation of BCG for the treatment of bladder cancer.
Laboratory Findings
Nonspecific.
MYCOBACTERIUM MARINUM (CLASSIC FISH TANK GRANULOMA)
M marinum is an acid-fast bacillus that is in Runyon class I, the photochromogens. It grows optimally at 32 °C and inhabits water and marine organisms. Infection of humans generally occurs after a trauma that takes place in water (eg, fish spines, nips by crustaceans); it can be acquired through open skin that comes into contact with swimming pools, aquariums, domestic fish tanks, or stagnant bodies of water.
Clinical Findings
Signs and Symptoms
The typical lesions of M marinum infection, which can resemble cutaneous sporotrichosis, are small papules on the extremities that enlarge, turn blue-purple, and then suppurate and ulcerate. Dissemination is rare, although it can occur in immunocompromised hosts.
Laboratory Findings
A skin biopsy should be taken for culture. Pathology shows granulomatous inflammation, but it is rare to find acid-fast bacilli on stain.
Differential Diagnosis
M marinum can resemble sporotrichosis.
Diagnosis
The diagnosis of atypical mycobacterial infections may be difficult to make with certainty. It is often unclear whether the presence of an atypical mycobacterium in a clinical specimen indicates infection or colonization. The diagnosis of an infection with mycobacteria should be made only in the presence of an illness associated with mycobacteria and when other causes of disease have been excluded.
Another clue that helps distinguish colonization from infection is the quantity of growth in culture. Heavy growth of one organism in culture is more suggestive of infection than of colonization; light growth is suggestive of colonization, unless the organism was isolated from a normally sterile body fluid. It is often helpful to notify the microbiology laboratory when infection with an atypical mycobacterium is suspected, because the nontuberculous mycobacteria often have very specialized culture requirements.
Experience is needed for accurate interpretation of the appearance of mycobacteria in stained specimens. Typically, fluorochrome, Kinyoun, and Ziehl-Neelsen stains are used. Cultures are incubated at 37 °C in 10% carbon dioxide and 90% air, usually for = 6-8 weeks. (More detailed information regarding laboratory diagnosis can be found in a standard microbiology text.) Typically, detection of growth in culture can be made within 2 weeks, but identification and speciation may take = 2-4 weeks. Susceptibility testing has not been standardized, and all significant isolates need to be tested for antibiotic susceptibility. However, susceptibility to an antibiotic in vitro has not been directly correlated to clinical efficacy.
Diagnosis of MAC, M bovis, and M marinum infections is determined by acid-fast bacillus smear and culturing from the infected tissue. Diagnosis of M scrofulaceum infection is made by identification of granulomatous inflammation in skin biopsy and isolation of the organism from culture. Diagnosis of M kansasii infection is difficult, especially since there can be a concurrent infection with M tuberculosis.
Diagnosis of leprosy depends on clinical information as well as biopsy of affected dermis; viable organisms stain brightly and uniformly (see Table 3). Nonviable organisms stain in an irregular manner. A skin biopsy taken from a patient with suspected leprosy should be stained with hematoxylin and eosin, as well as with Fite stain for acid fastness.
The two polar forms of leprosy are the lepromatous, or multibacillary, and the tuberculoid, or paucibacillary (see Box 3 for features of both). Most patients will have intermediate forms. In lepromatous patients, skin biopsies should be taken at sites of skin lesions, but normal looking skin will also have pathologic changes. Lesions will show many bacilli in clumps and foam cells loaded with bacilli in the dermis. Granulomatous changes may be seen in the liver, spleen, and lymph nodes. In tuberculoid patients, biopsies should be done only at sites of lesions because biopsies of normal appearing skin will be nondiagnostic. Skin biopsies from tuberculoid patients will show few or no organisms but will show granulomas of epithelial cells, lymphocytes, and foreign-body giant cells near dermal appendages, especially dermal nerves. The pathognomic lesion for tuberculoid leprosy is acute granulomatous invasion and destruction of dermal nerves. Most patients, however, have intermediate, or borderline, forms of leprosy, and their biopsies will show a mixture of findings.
An intradermal skin test is available that uses heat-killed M leprae. However, use of the skin test to make the diagnosis of leprosy is unreliable. Although the skin test will be positive in tuberculoid patients, treated lepromatous patients and unaffected individuals in endemic areas will show positive results as well. Lepromatous patients will be anergic specifically to M leprae but will have a normal response to intradermal recall antigens, such as purified protein derivative. Various M leprae lipid and carbohydrate constituents are believed to impair macrophage and T-lymphocyte function specifically against M leprae. There also seems to be an increase in the number of T-suppressor cells. Cytokines such as interleukin 2 (IL-2), interferon ? (IFN-?), IL-4, IL-5, and IL-10 are also thought to play a role in expression of disease.
Treatment of Atypical Mycobacterial Infections
MAC Infection. Treatment of pulmonary disease, disseminated disease, subcutaneous infections, and bone infections in immunocompetent patients consists of a regimen of three drugs (Box 4). Clarithromycin at a dose of 500 mg by mouth two times a day is given with ethambutol at a dose of 15-25 mg per kg by mouth every day. These two drugs are also then given with rifabutin, 300 mg by mouth every day for = 24 months. If there is an isolated lung lesion, surgical removal of the nodule is a treatment option. Other second-line drugs include azithromycin, clofazimine, ciprofloxacin, and amikacin. The regimen used should be tailored to in vitro susceptibility tests. Patients with pulmonary disease should have sputum samples tested every month during the course of treatment. In 80-90% of patients, the sputum should convert to negative in 1-2 months. The therapy should be extended for 12 months after the sputum conversion.
HIV-positive patients with disease can be treated with a three-drug regimen that includes clarithromycin or azithromycin, ethambutol, and/or rifabutin (Box 5). An alternative regimen includes clarithromycin or azithromycin, ethambutol, plus/ minus rifabutin and one of the following: ciprofloxacin, ofloxacin, or amikacin. A recent study of treatment regimens showed that therapy with rifabutin, ethambutol, and clarithromycin resulted in a longer mean survival than did therapy with rifampin, ethambutol, clofazimine, and ciprofloxacin. The efficacy of these regimens in children with HIV infection has not been well established.
Once immunosuppressed patients with AIDS are treated, they must be placed on lifelong suppressive therapy that includes clarithromycin or azithromycin plus ethambutol. Alternative drugs include clarithromycin or azithromycin alone or rifabutin.
AIDS patients who do not have documented active MAC disease and whose CD4 count is < 100 may be placed on primary prophylactic therapy, which consists of a single drug (Box 6). Those drugs used in primary prophylaxis are clarithromycin, azithromycin, or rifabutin. Clarithromycin reduces the MAC infection rate by 68% and was associated with a 38% mortality rate as compared with 47% with placebo.
M leprae Infection. Leprosy generally requires a long duration of treatment, and compliance is a major problem. Lepromatous leprosy requires a longer treatment time course than tuberculoid leprosy because of the greater number of organisms involved. Currently, the recommended treatment is dapsone and rifampin for 6 months for tuberculoid leprosy (Box 7). Dapsone alone is not recommended because of reports of emerging resistance. For lepromatous leprosy, dapsone with rifampin or clofazimine for 24 months is recommended. Nonetheless, there have been reports of relapses even after such long courses of treatment. Other agents such as ethionamide, prothionamide, the aminoglycosides, minocycline, clarithromycin, and the fluoroquinolones may also prove to be beneficial.
M kansasii Infection. Response to antimycobacterial therapy tends to be poor, but there have been reports of clinical responses. Treatment must be tailored to each individual patient depending on the in vitro sensitivities of M kansasii and whether immunosuppression is present. Treatment should consist of a three- to five-drug regimen depending on sensitivities. A common regimen for M kansasii includes rifampin (10 mg/kg/d for a maximum of 400 mg/d), isoniazid (5 mg/kg/d for a maximum of 300 mg/d), and ethambutol (15-25 mg/kg/d). Treatment should be continued for ~ 18 months and has been shown to have about a 90% response rate.
M kansasii has been shown to be sensitive in vitro to clarithromycin, erythromycin, amikacin, and the fluoroquinolones. These medications, along with sulfamethoxazole or bactrim, are second-line drugs. If lymphadenitis is present, the lymph node should be totally excised. There is no rationale for incision and drainage. In AIDS patients with disseminated disease, treatment should be extended for 15 months after cultures are negative. Treatment regimens in children have not been well established.
M scrofulaceum Infection. Antituberculosis drugs are not helpful. The treatment of choice is surgical excision of involved nodes. Clarithromycin may be useful in patients not responsive to excision.
M bovis Infection. Treatment of M bovis is the same as for M tuberculosis, although M bovis is uniformly resistant to pyrazinamide.
M marinum Infection. Treatment of M marinum infection consists of rifampin and ethambutol.
Prevention & Control of Atypical Mycobacterium Infection
No isolation measures are needed since the organisms are found in the environment. Prophylaxis is needed only in AIDS patients with MAC (see Box 6).
Table 1. Mycobacterium species, their habitats, and the diseases they cause.
Mycobacterium species
Habitat
Common Diseases in Humans
Tuberculosis complex
M tuberculosis
M bovis
Photochromogens
M kansasii
M marinum
M simiae
M asiaticum
Scotochromogens
M scrofulaceum
M szulgai
M gordonae
M flavescens
M xenopi
Nonchromogens
M avium-intracellulare
M ulcerans
M gastri
M terrae
Rapid growers
M fortuitum
M abscessus
M chelonae
M smegmatis
M leprae
- Humans
- Humans, cattle
- Water, cattle
- Fish, water
- Primates
- Primates
- Soil, water, foodstuffs
- Unknown
- Water
- Soil, water
- Water
- Soil, water, swine, cattle, birds
- Unknown
- Soil, water
- Soil, water
- Soil, water, animals, marine life
- Soil, water, animals, marine life
- Soil, water, animals, marine life
- Moist surfaces, urogenital flora
- Humans, nine-branded armadillos
- Bronchopulmonary
- Soft tissue & gastrointestinal tract
- Skeletal
- Skin and soft tissue
- Bronchopulmonary
- Pulmonary (rare)
- Lymphadenitis
- Bronchopulmonary
- Pulmonary (rare)
- Pulmonary (rare)
- Bronchopulmonary
- Pulmonary, lymphadenitis, disseminated
- Skin and soft tissue
- Pulmonary (rare)
- Pulmonary (rare)
- Skin, soft tissue, disseminated
- Skin, soft tissue, disseminated, skeletal
- Skin, soft tissue, disseminated, skeletal
- Pulmonary (rare)
- Skin, soft tissue, disseminated (rare)
Table 2. Clinical findings in Mycobacterium avium complex infection.1
Acid-fast bacillus, nonphotochromogen
Slow grower (10-21 days) at 36°C
CXR findings vary from small thin-walled cavities to lobar in filtrates and isolated nodules.
Diagnosis clinically:
- Presence of pulmonary cavities not attributable to another disease and 2 or more positive sputum or bronchial wash specimens with moderate to heavy growth of MAC
- Infiltrate present but no cavities present, with failure of sputum cultures to convert to negative with either pulmonary toilet or 2 weeks of specific antimicrobial therapy
- Lung biopsy with histological confirmation and positive culture
- One positive blood culture
- Isolation of MAC from any other sterile body site
- Positive bone marrow, lymph node, or liver biopsy
Table 3. Clinical findings in Mycobacterium leprae infection.
- Acid-fast bacillus, Gram stain variable
- Presence of dopaoxidase activity, loss of acid fastness by pyridine extraction
- Multiplies in mouse foot with doubling time of 12 d
- Hematoxylin and eosin stain of skin biopsy shows organisms; sometimes silver stain positive
BOX 1. MAC in Immunocompetent Patients
Children
Adults
More Common
- Superficial lymphadenitis
- Cutaneous ulcers, abscesses and plaques
- Colonization
- Pulmonary infection — productive cough hemoptysis in < 25%, fever and weight loss in 33%
Less Common
- Pulmonary
- Disseminated disease
- Cutaneous lesions
- Lymphadenitis
- Disseminated disease
BOX 2. MAC in AIDS Patients (CD4 < 100)
Children
Adults
More Common
- Disseminated disease
- Pulmonary disease
- Colonization-gastrointestinal and respiratory tracts
- Fever, drenching night sweats, weight loss, diarrhea, abdominal pain, anorexia, hepatosplenomegaly, adenopathy, and anemia
Less Common
- Isolated lymphadenitis
- Isolated cutaneous lesions
- Pulmonary infection
- Osteomyelitis, peritonitis, oral lesions, appendicitis
BOX 3. Mycobacterium leprae Syndromes
Lepromatous Forms
Tuberculoid Forms
- Symmetrical skin plaques, nodules, or thickened dermis, usually on cooler parts of the body
- Infiltration of upper respiratory system and nasal cartilage, resulting in “saddle nose” deformity
- Symmetric and generalized peripheral neuropathy
- Diffuse lepromatosis seen in Mexican patients, diffuse dermal infiltration without focal lesions
- Normal-looking skin will show organisms in clumps, foam cells in deep dermis, and granulomas in liver, spleen, and lymph nodes
- Few hypopigmented anesthetic macules with distinct elevated and erythematous borders
- Large and asymmetric peripheral nerve involvement
- Neural leprosy causes large functionally impaired nerve trunks without skin lesions
- Lesions and rims should be biopsied (normal-looking skin will not have abnormalities); granulomas with invasion and destruction of dermal nerves
BOX 4. Treatment of MAC in Immunocompetent Patients
Adults
Children
First Choice
Clarithromycin 500 mg PO twice daily + ethambutol 15-25 mg/kg PO + rifabutin 300 mg PO once daily for as many as 24 months, treat for at least 12 months after sputum conversion; surgical excision if one isolated pulmonary nodule
Clarithromycin, 7.5 mg/kg PO twice daily (max. dose 500 mg/dose) in children > 6 months old + ethambutol, 15 mg/kg/d as a single dose in children > 13 years old + rifabutin
Second Choice
Rifampin + ethambutol + clofazamine + ciprofloxacin, 750 mg PO twice daily
BOX 5. Treatment of MAC in AIDS patients
First Choice
Same regimen as adult treatment of MAC in immunocompetent patients. May substitue azithromycin (500 mg once daily) for clarithromycin
Second Choice
Clarithromycin or azithromycin + ethambutol + rifabutin + 1 or more of the following
- Ciprofloxacin, 750 mg PO twice daily
- Ofloxacin, 400 mg PO twice daily
- Amikacin, (7.5-15 mg/kg) IV or IM
Pediatric Considerations
Treatment regimens for pediatric AIDS patients are not well established.
BOX 6. Control of MAC Infection in AIDS Patients
Prophylactic Measures
- Lifelong prophylaxis in AIDS patients with CD4 < 100: clarithromycin, 500 mg PO twice daily or azithromycin, 1200 mg PO weekly, or rifabutin, 300 mg PO once daily.
- Secondary prophylaxis in AIDs patients after treatment of MAC disease: clarithromycin OR azithromycin PLUS ethambutol (15 mg/kg/d)
Isolation Precautions
- None
BOX 7. Treatment of Mycobacterium leprae
Adults
Children
First Choice: Tuberculoid
Dapsone, 100 mg once daily unsupervised + rifampin, 600 mg once per month supervised for 6 months
Correspondingly lower dose
First Choice: Lepromatous
Dapsone, 100 mg once daily + clofazamine, 50 mg once daily unsupervised OR rifampin, 600 mg once daily + clofazamine, 300 mg every month supervised for 24 months
Correspondingly lower doses
Alternative Choice
Ethionamide, 250 mg once daily or prothionamide, 375 mg once daily in place of clofazamine; minocycline, clarithromycin, or fluoroquinolone reportedly bactericidal