Definition
Table Centers for Disease Control and Prevention 1993 Revised Classification System for HIV Infection in Adults and AIDS Surveillance Case Definition and Table Centers for Disease Control and Prevention 1994 Revised Classification System for HIV Infection in Children Younger than 13 Years present the revised classification systems for adult and child HIV infection.
Pathogenesis
Transmission of HIV
- Infection with HIV occurs through three primary modes: sexual, parenteral, and perinatal. Sexual intercourse, primarily receptive anal and vaginal intercourse, is the most common vehicle for transmission. The probability of HIV transmission from receptive anorectal intercourse is 0.1% to 3% per sexual contact and 0.1% to 0.2% per sexual contact for receptive vaginal intercourse. In general, the risk is increased when the index partner is in an advanced stage of disease. Persons at higher risk for heterosexual transmission include those with ulcerative sexually transmitted diseases, those with multiple sex partners, and sexual partners of intravenous drug users.
- The use of contaminated needles or other injection-related paraphernalia by drug abusers has been the main cause of parenteral transmissions and currently accounts for one-fourth of AIDS cases reported in the United States.
- Health care workers have a small risk of occupationally acquiring HIV, mostly through needlestick injury.
- Perinatal infection, or vertical transmission, is the most common cause (greater than 90%) of pediatric HIV infection. The risk of mother-to-child transmission is approximately 25% in the absence of breast feeding or antiretroviral therapy. Breast feeding can also transmit HIV.
Clinical presentation
- Clinical presentations of primary HIV infection vary, but patients often have a viral syndrome or mononucleosis-like illness with fever, pharyngitis, and adenopathy (Table Clinical Presentation of Primary HIV Infection in Adults). Symptoms may last for 2 weeks.
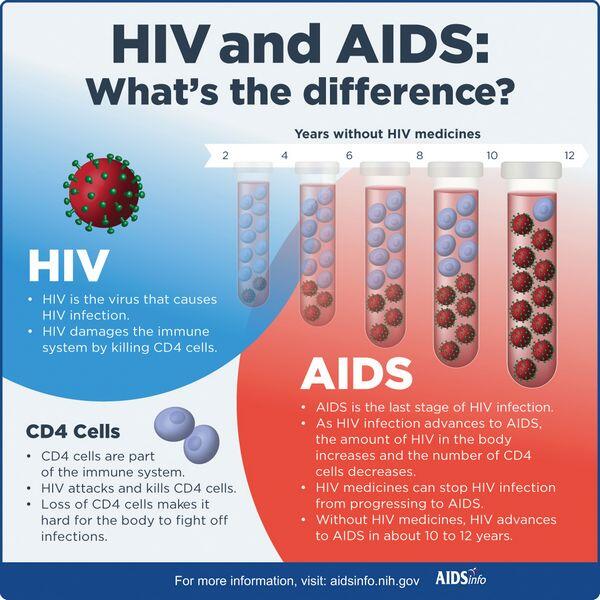
- Probability of progression to AIDS is related to RNA viral load; in one study, 5-year progression rates to AIDS were 8%, 26%, 49%, and 62% for RNA copies per milliliter of less than 4530, 4531 to 13,020, 13,021 to 36,270, and greater than 36,270, respectively.
- The classification scheme of the Centers for Disease Control and Prevention divides HIV infection into a matrix of nine categories based on the CD4 cell count (see «Diagnosis» below) and clinical conditions (see Table Centers for Disease Control and Prevention 1993 Revised Classification System for HIV Infection in Adults and AIDS Surveillance Case Definition).
- Most children born with HIV are asymptomatic. On physical examination, they often present with unexplained physical signs such as lymphadenopathy, hepatomegaly, splenomegaly, failure to thrive and weight loss or unexplained low birth weight, and fever of unknown origin. Laboratory findings include anemia, hypergammaglobulinemia, altered mononuclear cell function, and altered T-cell subset ratios. The normal range for CD4 cell counts in children is much different than for adults (Table Centers for Disease Control and Prevention 1994 Revised Classification System for HIV Infection in Children Younger than 13 Years).
- Clinical presentations of the opportunistic infections are presented in «Infectious Complications of HIV,» below.
| TABLE. Centers for Disease Control and Prevention 1993 Revised Classification System for HIV Infection in Adults and AIDS Surveillance Case Definition | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| TABLE. Centers for Disease Control and Prevention 1994 Revised Classification System for HIV Infection in Children Younger than 13 Years | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| TABLE. Clinical Presentation of Primary HIV Infection in Adults | |
|
Diagnosis
- The most commonly used screening method for HIV is an enzyme-linked immunosorbent assay (ELISA), which detects antibodies against HIV-1 and is both highly sensitive and specific. False positives can occur in multiparous women; in recent recipients of hepatitis B, HIV, influenza, or rabies vaccine; following multiple blood transfusions; and in those with liver disease or renal failure, or undergoing chronic hemodialysis. False negatives may occur if the patient is newly infected and the test is performed before antibody production is adequate. The minimum time to develop antibodies is 3 to 4 weeks from initial exposure.
- Positive ELISAs are repeated in duplicate and if one or both tests are reactive, a confirmatory test is performed for final diagnosis. Western blot assay is the most commonly used confirmatory test.
- The viral load test quantifies viremia by measuring the amount of viral RNA. There are several methods used for determining the amount of HIV RNA: reverse transcriptase-coupled polymerase chain reaction (RT-Polymerase chain reaction), branched DNA (bDNA), and transcription-mediated amplification. Each assay has its own lower limit of sensitivity, and results can vary from one assay method to the other; therefore, it is recommended that the same assay method be used consistently within patients.
- Viral load can be used as a prognostic factor to monitor disease progression and the effects of treatment.
- The number of CD4 lymphocytes in the blood is a surrogate marker of disease progression. The normal adult CD4 lymphocyte count ranges between 500 and 1600 cells/ВµL, or 40% to 70% of all lymphocytes.
Treatment of HIV/AIDS
Infectious complications of HIV
- The development of certain opportunistic infections is directly or indirectly related to the level of CD4 lymphocytes.
- The most common opportunistic diseases and their frequencies found before death in patients with AIDS between 1990 and 1994 were Pneumocystis carinii pneumonia, Mycobacterium avium complex, and cytomegalovirus disease.
- The spectrum of infectious diseases observed in HIV-infected individuals and recommended first-line therapies are shown in Table Therapies for Common Opportunistic Pathogens in HIV-Infected Individuals.
Pneumocystis carinii
P. carinii pneumonia is the most common life-threatening opportunistic infection in patients with AIDS. The taxonomy of the organism is unclear, having been classified as both protozoan and fungal.
Clinical Presentation
- Characteristic symptoms include fever and dyspnea; clinical signs are tachypnea, with or without rales or rhonchi, and a nonproductive or mildly productive cough. Chest radiographs may show florid or subtle infiltrates or may occasionally be normal, although infiltrates are usually interstitial and bilateral. Arterial blood gases may show minimal hypoxia (PaO2 80 to 95 mm Hg) but in more advanced disease may be markedly abnormal.
- The onset of P. carinii pneumonia is often insidious, occurring over a period of weeks, although more fulminant presentations can occur.
| TABLE. Therapies for Common Opportunistic Pathogens in HIV-Infected Individuals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Treatment
- Treatment with trimethoprim-sulfamethoxazole or parenteral pentamidine is associated with a 60% to 100% response rate. Trimethoprim-sulfamethoxazole is the regimen of choice for treatment and subsequent prophylaxis of P. carinii pneumonia in patients with and without HIV.
- Trimethoprim-sulfamethoxazole is given in doses of 15 to 20 mg/kg/day (based on the trimethoprim component) as three to four divided doses for the treatment of P. carinii pneumonia.
- Trimethoprim-sulfamethoxazole is usually initiated by the intravenous route, although oral therapy (as oral absorption is high) may suffice in mildly ill and reliable patients or to complete a course of therapy after a response has been achieved with intravenous administration.
- For treatment of HIV-associated P. carinii pneumonia, pentamidine isethionate is administered intravenously, usually in doses of 4 mg/kg /day.
- The optimum length of therapy for treatment of P. carinii pneumonia with either agent is not known, but 21 days is commonly recommended.
- The more common adverse reactions seen with trimethoprim-sulfamethoxazole are rash, fever, leukopenia, elevated serum transaminases, and thrombocytopenia. The incidence of these adverse reactions is higher in HIV-infected individuals than in those not infected with HIV.
- For pentamidine, side effects include hypotension, tachycardia, nausea, vomiting, severe hypoglycemia or hyperglycemia, pancreatitis, irreversible diabetes mellitus, elevated transaminases, nephrotoxicity, leukopenia, and cardiac arrhythmias.
- The early addition of adjunctive glucocorticoid therapy to anti-P. carinii pneumonia regimens has been shown to decrease the risk of respiratory failure and improve survival in patients with AIDS and moderate to severe P. carinii pneumonia (PaO2 less than or equal to ≤ 0 mm Hg or [A-a] gradient ≥ reater than or equal to 35 mm Hg). The regimen currently recommended is 40 mg of prednisone orally twice daily during days 1 through 5; 40 mg once daily on days 6 through 10; and 20 mg once daily on days 11 through 21, or for the duration of therapy. In general, adjunctive glucocorticoid therapy should be initiated when antipneumocystis therapy is started, as the data supporting the use of glucocorticoids are based on initiation within the first 24 to 72 hours of the start of antipneumocystis therapy.
Prophylaxis
- Currently, P. carinii pneumonia prophylaxis is recommended for all HIV-infected individuals who have already had previous P. carinii pneumonia. Prophylaxis is also recommended for all HIV-infected persons who have a CD4 lymphocyte count of less than 200 cells/ВµL (i.e. their CD4 cells are less than 20% of total lymphocytes), unexplained fever (greater than 100В° F) for more than 2 weeks, or a history of oropharyngeal candidiasis. Patients on P. carinii pneumonia prophylaxis whose CD4 counts increase above 200 cells/ВµL antiretroviral therapy should not discontinue P. carinii pneumonia prophylaxis at this point (Table 39-7).
- Trimethoprim-sulfamethoxazole is the preferred therapy for both primary and secondary prophylaxis of P. carinii pneumonia in adults and adolescents. The recommended dose in adults and adolescents is one double-strength tablet daily.
- Trimethoprim-sulfamethoxazole is also the recommended drug of choice for P. carinii pneumonia prophylaxis in children. The trimethoprim-sulfamethoxazole regimen recommended (although other acceptable alternatives exist) is 150 mg/m2/day of trimethoprim and 750 mg/m2/day of sulfamethoxazole given in divided doses twice daily, 3 times weekly on consecutive days (e.g., Monday, Tuesday, and Wednesday). The total daily dose of trimethoprim-sulfamethoxazole in children should not exceed 320 mg of trimethoprim with 1600 mg of sulfamethoxazole.
Toxoplasma gondii
T. gondii can infect any organ of the body and cause an acute infection; it has a predilection for the brain and the eye.
Clinical Presentation
- The clinical signs and symptoms of toxoplasmosis are most frequently associated with involvement of the central nervous system and, less commonly, the lungs and eyes, although any organ can be affected. Clinical presentation often includes fever, headache, seizures (in approximately 10% to 25% of patients), focal neurologic abnormalities (in approximately 60% to 90%), and mental status changes.
- Brain biopsy is required to make a definitive diagnosis of toxoplasmic encephalitis, although presumptive diagnosis is commonly made in T. gondii-seropositive patients with typical central nervous system lesions.
Treatment
- The initial treatment of central nervous system toxoplasmosis is usually empiric. Antitoxoplasmosis therapy is usually initiated in patients with AIDS who are seropositive for Toxoplasma, have clinical symptoms suspicious for toxoplasmosis, and have characteristic findings on neuroradiographic studies.
- The combination of pyrimethamine and sulfadiazine is considered the most effective regimen for acute therapy of AIDS-related central nervous system toxoplasmosis.
- Pyrimethamine loading doses of 75 mg orally on the first day followed by 25 mg/day thereafter have been commonly used. Others have recommended larger loading doses of 100 to 200 mg followed by daily oral doses of 1 to 1.5 mg/ kg/day (50 to 100 mg/day).
- The usual dose of sulfadiazine is 1 to 1.5 g every 6 hours (4 to 8 g/day).
- Folinic acid, in doses of 10 to 20 mg/day (although doses as high as 50 mg/day have been used) is usually added to the combination to reduce the pyrimethamine-induced bone marrow toxicity.
- Acute therapy with this combination should be continued for at least 3 weeks, but 6 weeks of treatment is recommended for more severely ill patients.
- The combination of pyrimethamine and clindamycin appears to be less toxic, but less effective, than that of pyrimethamine and sulfadiazine.
- The discontinuation of pyrimethamine and sulfadiazine after successful initial therapy may be considered in persons who have a sustained CD4 cell count of greater then 200 cells/ВµL, for at least 6 months and have completed initial therapy and are asymptomatic.
- A regimen of pyrimethamine (25 to 50 mg/day with leukovorin 10 to 25 mg orally once dialy) and sulfadiazine (500 to 1000 mg four times daily) is recommended for maintenance therapy.
Cryptococcus neoformans
Cryptococcal infection is now uncommon and occurs primarily in those with limited access to health care.
Clinical Presentation
- The usual clinical presentation of cryptococcal infection is meningitis. The clinical features of cryptococcal meningitis may be subtle, nonspecific, and not localized to the central nervous system. Fever, headache, and malaise are the most frequent symptoms.
| TABLE. Therapies for Prophylaxis of First Episode Opportunistic Diseases in Adults and Adolescents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||
- Methods for diagnosis of cryptococcal infection include serum and cerebrospinal fluid (cerebrospinal fluid) testing for cryptococcal antigen and fungal cultures. Detection of cryptococcal antigen in serum and cerebrospinal fluid is the most sensitive and specific test; an antigen titer greater than 1:8 should be regarded as evidence of infection.
Treatment
- The standard therapeutic approach has been amphotericin B for both acute and maintenance therapy.
- Fluconazole is also effective for treatment of cryptococcal meningitis; however, the combination of amphotericin B and flucytosine was found to be superior.
- Most patients with cryptococcal meningitis should receive amphotericin B in an intravenous dose of at least 0.5 mg/kg/day for a minimum of 2 weeks as acute therapy. Flucytosine in doses of 100 to 150 mg/kg/day can be considered for combination with amphotericin B.
- Serum concentrations of flucytosine should be monitored, and peak levels kept below 100 mcg/mL to minimize hematologic adverse reactions.
- Maintenance therapy is necessary to prevent relapse. Fluconazole is the drug of choice to prevent relapse of cryptococcal meningitis.
Mycobacterium infections
Clinical Presentation
- The clinical syndrome associated with Mycobacterium avium complex (Mycobacterium avium complex) includes high spiking fevers, diarrhea, night sweats, malaise, weight loss, anemia, and neutropenia. Persistent diarrhea and abdominal pain, a malabsorption syndrome, and extrahepatic biliary obstruction are manifestations associated with Mycobacterium avium complex gastrointestinal infection.
- Diagnosis of Mycobacterium avium complex infection is usually based on culture of the organisms from the blood, although biopsies of the liver, bone marrow, and lymph nodes are also highly sensitive and specific.
Treatment
- Treatment regimens should contain at least two antimycobacterial agents.
- Every regimen should contain either clarithromycin or azithromycin, with clarithromycin (500 mg twice daily) being the preferred agent. For the second agent, numerous choices are available, although ethambutol (15 mg/kg/day) is preferred by many experts. Many clinicians would add a third (such as rifabutin), and some, a fourth drug to this regimen.
- Clinical responses usually occur within 2 to 8 weeks of the start of therapy. If a clinical and microbiologic response is observed, therapy should continue for the duration of the patient’s life.
- Mycobacterium avium complex prophylaxis is now strongly recommended for all HIV-infected adults and adolescents with a CD4 count less than 50 cells/ВµL. The first-line choices are either azithromycin (1200 mg once weekly) or clarithromycin (500 mg twice daily). Rifabutin is an alternative.
Herpes virus infections
Herpes Simplex Virus
- The manifestations of Herpes Simplex Virus disease observed in persons with AIDS include orolabial, genital, and anorectal mucocutaneous disease; esophagitis; and less commonly, encephalitis. Ulcerative Herpes Simplex Virus lesions present for longer than 1 month in an individual with laboratory evidence of HIV infection, or no other apparent cause for immunodeficiency, are considered an AIDS-defining condition.
- Symptoms of anorectal lesions, the most common clinically evident Herpes Simplex Virus disease causing morbidity in homosexual men, include pain, itching, and painful defecation.
- Acyclovir is the drug of choice for treatment of Herpes Simplex Virus disease. For mild to moderate mucocutaneous disease, oral acyclovir in doses of 200 mg five times daily or 400 mg three times daily are used, although 400 mg five times daily may be necessary. Intravenous acyclovir (15 mg/kg/day) should be used in those settings where absorption of an oral drug is questionable, oral tolerance is unlikely (Herpes Simplex Virus esophagitis), or perhaps when severe mucocutaneous disease is present.
- Treatment of mucocutaneous disease should be continued until all lesions have crusted.
- Intravenous acyclovir (30 mg/kg/day) should also be used for viscerally disseminated disease and for Herpes Simplex Virus encephalitis.
- Recurrent Herpes Simplex Virus disease can often be managed with low-dose suppressive oral acyclovir therapy, 200 mg four times daily, 400 mg twice daily, or 800 mg once daily.
Varicella-Zoster Virus
- Zoster usually begins as radicular pain followed by localized erythematous rash and characteristic vesicles. Zoster will usually remain confined to a limited number of dermatomes, but complications such as widespread cutaneous involvement and disseminated visceral zoster may occur.
- Acyclovir is the drug of choice for Varicella zoster virus infections. While an oral acyclovir regimen of 4 g/day has been shown effective for the treatment of zoster in immunocompetent adults, the drug has not been fully evaluated in immunocompromised patients such as those with AIDS.
- AIDS patients with disseminated cutaneous or visceral zoster should receive treatment with intravenous acyclovir in doses of 30 mg/kg/day for at least 7 days or until all lesions are crusted.
Cytomegalovirus
- Manifestations of cytomegalovirus infection include retinitis, esophagitis, hepatitis, gastrointestinal involvement, and less commonly radiculopathy, encephalitis, and pneumonitis. cytomegalovirus retinitis is usually associated with a painless progressive loss of vision. Patients may initially complain of blurry vision, loss of visual acuity, or «floaters.»
- Ganciclovir therapy for cytomegalovirus disease has traditionally been divided into two phases – induction and maintenance – because high relapse rates are found after discontinuation of the drug following successful completion of a 2- to 3-week course of initial therapy. Induction regimens are typically 7.5 to 10 mg/kg/day intravenously in two or three equally divided doses for 14 days, or longer if there is a slow clinical response. Maintenance therapy is usually 5 to 6 mg/kg once daily, although doses of 10 mg/kg have been used, 5 to 7 days/wk for an indefinite period of time.
- Foscarnet is an alternative to ganciclovir that appears less likely to cause neutropenia; however, it has a variety of potential adverse effects, including renal insufficiency and metabolic disturbances (both increases and decreases) in calcium and phosphorus.
- Prophylaxis with oral ganciclovir should be considered in HIV-infected adults and adolescents who have a CD4 cell count of less than 50 cells/ВµL; ganciclovir prophylaxis is not a recommended standard of care.