Central nervous system infections, especially bacterial meningitis, are frequently life-threatening and usually constitute medical emergencies that require accurate and prompt treatment. (Portions of this section about meningitis have previously been published [Swartz and O’Hanley 1987] and are reproduced with permission of the publisher, Scientific American Medicine, New York. [Send permission to section editor.]) Fortunately, advances in methods of diagnosis and treatment developed during the past 15 years have significantly improved the prognosis associated with many of these illnesses. New diagnostic methods (such as latex agglutination and polymerase chain reaction) supplement rather than supplant cerebrospinal fluid studies. The cerebrospinal fluid studies frequently provide important initial information needed for clinical and microbiologic diagnosis; when not definitive, they serve at least to focus the differential diagnosis. New chemotherapeutic agents, both antibacterial (e.g., the third-generation cephalosporins) and antiviral (e.g., acyclovir), are directly responsible for this improvement. The use of adjunctive therapy with glucocorticoids may lessen some of the adverse pathophysiologic consequences of bacterial meningitis.

Etiologic agents
Meningitis used to be a disease that occurred primarily in children younger than 12 years. The advent of a vaccine for Haemophilus influenzae has led to a marked change in the epidemiology of meningitis in developed countries. In the United States in 1995, on the basis of active surveillance in 22 counties representing 3.9% of the U.S. population, 5755 cases of bacterial meningitis occurred, a reduction of 55% from 1986 when 12,920 cases occurred. Furthermore, the median age of persons with bacterial meningitis increased from 15 months in 1986 to 25 years in 1995, chiefly because of a 94% reduction in the number of cases of H. influenzae meningitis. In 1995 the meningitis rates per 100,000 population for the following pathogens were as follows: Streptococcus pneumoniae, 1.1; Neisseria meningitidis, 0.6; group B streptococcus, 0.2; Listeria monocytogenes, 0.2; and H. influenzae, 0.2. The principal bacteria responsible for neonatal meningitis are gram-negative bacilli (usually E. coli strains bearing the K1 capsular antigen) and group B streptococci. Streptococcus pneumoniae is now the most common cause of meningitis beyond the neonatal period. The appearance of H. influenzae meningitis in adults is so unusual that its presence suggests predisposing anatomic or immunologic defects that have permitted circumvention of the barrier normally provided by serum bactericidal mechanisms. The bacterial agents that most frequently cause bacterial meningitis in adults are S. pneumoniae and N. meningitidis.
| Table Cost of Various Antibiotics Used to Treat Febrile Neutropenic Patients | ||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
Other bacteria infrequently cause meningitis. Listeria monocytogenes is a rare pathogen in the neonate. Its involvement in adult cases has been increasing primarily among those who are immunosuppressed or elderly. Isolation of an anaerobic organism from the cerebrospinal fluid is rare and strongly suggests intraventricular leakage of a brain abscess or the presence of a parameningeal focus of infection. Staphylococcus aureus meningitis is associated with neurosurgical procedures, penetrating cranial trauma, staphylococcal bacteremia and endocarditis, immunosuppressive therapy, and underlying neoplastic disease. Meningitis complicating ventriculoatrial or ventriculoperitoneal shunting procedures is usually caused by S. aureus or S. epidermidis. Gram-negative bacillary meningitis (caused by E. coli, Enterobacter, Klebsiella, Proteus, Serratia, or Pseudomonas species) usually is a nosocomial infection that affects neurosurgical, immunosuppressed, and oncologic patients, the elderly, and neonates. A specific bacterial agent cannot be identified in 5% to 10% of patients with pyogenic meningitis. Polymicrobial mixed meningitis is rare and is found most often in neonates, particularly in association with a neuroectodermal defect, and occasionally in older patients following a penetrating head injury. Therefore, the common rule is that suppurative bacterial meningitis is caused by a single aerobic bacterial pathogen. The advent of increasing resistance of Streptococcus pneumoniae to penicillin has altered our approach to the treatment of meningitis beyond the neonatal period. If there is ≥2% rate of high-level penicillin-resistant S. pneumoniae in the community, empiric treatment of bacterial meningitis should consist of cefotaxime or ceftriaxone plus vancomycin. Ampicillin should be added if Listeria is suspected.
Principles
Vaccination with H. influenzae vaccine has markedly reduced the rate of childhood meningitis. To treat properly, the practitioner must be aware of the common bacterial pathogens (and their local antimicrobial susceptibility patterns) associated with suppurative meningitis in each age group.
Pathogenesis and clinical features
Pathogenic organisms may enter the meninges in several ways:
- From the bloodstream during bacteremia,
- By penetration directly from the nasopharynx (skull fracture, congenital dural defect, or eroding sequestrum in mastoid), or from the body surface (neuroectodermal defect),
- Through intracranial passage via nasopharyngeal venules,
- By direct spread from an adjacent focus of infection (sinusitis or intraventricular leakage of a brain abscess),
- By introduction of organisms at the time of a neurosurgical operation,
- By contamination of a cerebrospinal fluid drain
Bacteremia is the most frequent source of infection. Organisms often are demonstrable in the bloodstream in the very early stages of the three most common types of bacterial meningitis beyond the neonatal period: those caused by S. pneumoniae, N. meningitidis, and group B streptococcus. Once meningeal infection becomes established, it quickly extends throughout the subarachnoid space. Ventriculitis can be demonstrated at the time of admission in at least 70% of neonates who are diagnosed as having meningitis. Certain clinical conditions are predisposing factors for specific types of bacterial meningitis. The conditions that predispose a person to pneumococcal meningitis include acute otitis media and mastoiditis. These entities precede 30% of cases of meningitis. In adult patients, pneumonia precedes 10% to 25%; nonpenetrating head injury precedes 5% to 10%; cerebrospinal fluid rhinorrhea or otorrhea precedes 5%; and in adults, alcoholism and cirrhosis of the liver precede 10% to 25% of cases with pneumonococcal meningitis in urban hospitals. Sickle cell anemia, defects in host defenses such as congenital or acquired immunoglobulin deficiencies, asplenic states, and acute sinusitis occasionally precede meningitis. Most cases of H. influenzae N. meningitidis, and S. pneumoniae meningitis are preceded by upper respiratory tract infection, otitis media, or pneumonia. The onset is usually sudden and progresses over the course of 24 to 36 hours with fever, generalized headache, vomiting, and stiff neck. Myalgias (particularly in meningococcal disease) and backache are common. Once meningitic signs are evident, the infection progresses rapidly, producing confusion, obtundation, and ultimately coma. Indications of leptomeningeal inflammation (drowsiness, stiff neck, and Kernig and Brudzinski signs) are generally present. The usual manifestations of meningitis may be partially obscured in an elderly person who has underlying congestive heart failure or pneumonia and is obtunded and hypoxic. Similarly, a neonate with meningitis may have decreased appetite, fever, irritability, lassitude, and vomiting but may not always exhibit either stiff neck or bulging fontanelles. Such patients should be examined carefully for meningitic signs; if any question about the presence of meningitis remains in such cases, a lumbar puncture must be performed to obtain cerebrospinal fluid for examination. A number of neurologic findings and complications may accompany bacterial meningitis. These include cranial nerve dysfunction (especially third, fourth, sixth, and seventh nerves), focal cerebral signs (hemiparesis, dysphasia, and hemianopia), focal and generalized seizures, and acute cerebral edema, ultimately leading to death. The presence of skin lesions may assist the physician in arriving at a diagnosis. A maculopetechial or purpuric rash in a patient with meningitis usually signifies meningococcal infection. Infrequently, petechial and purpuric skin lesions develop in the course of S. pneumoniae bacteremia and meningitis, reflecting disseminated intravascular coagulation. Multiple skin lesions almost identical to those observed in patients with meningococcemia occur rarely in patients with acute S. aureus endocarditis. Meningitic signs and a cerebrospinal fluid neutrophilic pleocytosis also may develop in such patients and are caused by embolic cerebral infarction rather than by bacterial meningitis. The maculopetechial rash of echovirus aseptic meningitis (particularly type 9 that has been responsible for extensive outbreaks) may be mistaken for the rash of meningococcal meningitis. This type of viral meningitis may produce an early and marked cerebrospinal fluid neutrophilic pleocytosis. The rash in meningococcal meningitis may involve the face and neck but only after it has already extensively covered other parts of the body; in echovirus type 9 meningeal infection, the rash involves the face and neck early in the course of the exanthem before other parts of the body are significantly involved.
Laboratory features
The cerebrospinal fluid cell count in the majority of patients with untreated bacterial meningitis ranges from 100 to 5000 cells/mm3, of which more than 80% are neutrophils. Cell counts of 50,000 cells/mm3 or higher are occasionally observed in primary bacterial meningitis, but such a marked pleocytosis also suggests the possibility of intraventricular rupture of a cerebral abscess. More than half of patients with bacterial meningitis have glucose concentrations of 40 mg/dL or lower in their cerebrospinal fluid (<50 to 60% of the simultaneous fasting blood glucose concentration). A normal cerebrospinal fluid glucose concentration, however, can occur in some patients with bacterial meningitis. The primary importance of the cerebrospinal fluid determination of glucose is not in aiding the diagnosis of the typical case of acute pyogenic meningitis (which usually can be diagnosed on the basis of the gram-stained smear of the cerebrospinal fluid and the cerebrospinal fluid cell count). Rather, the cerebrospinal fluid glucose concentration is most useful in distinguishing chronic meningitides (such as those that are caused by Listeria, Nocardia, Actinomyces, Cryptococcus, or Coccidioides) marked by hypoglycorrhachia from parameningeal infections and viral aseptic meningitides, which usually do not lower cerebrospinal fluid glucose concentrations. Patients with bacterial meningitis usually have cerebrospinal fluid protein concentrations higher than 120 mg/dL (normal 30–40 mg/dL). Occasionally, values of 1000 mg/dL or greater are observed, suggesting actual or impending subarachnoid block secondary to the meningitis. Definitive diagnosis requires isolation of the causative organism or demonstration of its characteristic antigen, from the cerebrospinal fluid. The implicated bacterium can be demonstrated on a cerebrospinal fluid gram-stained smear in about 80% of patients with bacterial meningitis. The bacteria that are most likely to be missed on Gram stain are meningococci and Listeria species. Bacteremia is demonstrable in 80% of patients with H. influenzae meningitis, 50% with pneumococcal meningitis, and 30% to 40% with N. meningitidis meningitis. Cultures of cerebrospinal fluid and blood yield enough information to permit determination of the bacterial cause in about 90% of previously untreated patients with bacterial meningitis.
Principles
Definitive diagnostic studies are mandatory in any life-threatening disease. They confirm the clinical diagnosis, and the data derived from these tests provide rational guidelines for therapy. Common bacterial meningitides (those caused by S. pneumoniae, group B streptococcus, and previously Haemophilus influenzae) are associated with mean cerebrospinal fluid bacterial concentrations ranging from 105 to 107 organisms per milliliter. This concentration of organisms introduces envelope polysaccharide antigens into the cerebrospinal fluid in amounts sufficient to be detectable by counterimmunoelectrophoresis and the latex agglutination test. These procedures have been used most extensively in the rapid diagnosis (i.e., 1 to 2 hours) of H. influenzae meningitis. In addition, counterimmunoelectrophoresis has been helpful in the rapid diagnosis of pneumococcal and meningococcal (groups A, B, C, and Y) meningitis, and it can be used to detect E. coli Kl capsular antigen and group B streptococcal antigen in neonatal meningitis. The bacterial capsular antigens of the three common etiologic agents in primary bacterial meningitis can be successfully identified in 60 to 80% of cases. The reliability of these immunoprecipitation techniques depends heavily on the activity of the antisera employed. False-positive results occasionally arise from cross-reactions. For example, certain E. coli capsular antigens cross-react with antisera to H. influenzae type B and group B meningococcal antigens. Because in about 90% of patients with bacterial meningitis the bacterial cause can be established by more traditional microbiologic means, counterimmunoelectrophoresis is not essential for diagnosis. It serves as an important adjunct, however, permitting early diagnosis in patients in whom no organisms are seen on smear or in whom cultures remain negative because of prior therapy with antibiotics. Even in cases in which the morphology of the organism is revealed by gram-stained smears, counterimmunoelectrophoresis is useful because it permits definitive identification of the agent.
Radiologic studies
Roentgenograms of the chest, sinuses, and mastoids should be performed at an appropriate time following institution of antimicrobial therapy for suspected pyogenic meningitis; infections in these areas are frequently associated with meningitis. When history, clinical setting, or physical findings suggest the presence of a suppurative intracranial collection, such as a brain abscess or subdural empyema, computerized tomography scanning should be performed without delay. It is not appropriate or necessary to routinely perform a computerized tomography or radionuclide scan to exclude these diagnostic entities when the practitioner is confident of the diagnosis of uncomplicated suppurative meningitis. Meningitis itself induces the following changes on the computerized tomography scan: contrast enhancement of the leptomeninges and ventricular lining; widening of the subarachnoid space; and patchy areas of diminished density in the cerebrum from cerebritis and necrosis. In addition, computerized tomography scanning may be helpful in evaluating the patient with a prolonged or deteriorating clinical status and in detecting suspected complications, such as sterile subdural collections or empyema, ventricular enlargement secondary to communicating or obstructive hydrocephalus, ventriculitis or ventricular empyema (as revealed by ventricular wall enhancement), or cerebral infarction caused by arteritis or cortical vein thrombophlebitis.
Differential diagnosis of suppurative bacterial meningitis
Because the clinical features of bacterial meningitis (headache, fever, stiff neck, and obtundation) may be seen in other types of central nervous system infection, findings in the cerebrospinal fluid are important in the development of an appropriate differential diagnosis. Particular attention should be given to the patient with meningitic signs and neutrophilic cerebrospinal fluid pleocytosis, but normal cerebrospinal fluid glucose concentrations, or absence of organisms in a gram-stained smear of the cerebrospinal fluid. The differential diagnosis in such cases includes several treatable diseases that require management that is different from that of bacterial pyogenic meningitis. A parameningeal bacterial infection such as an epidural abscess, a subdural empyema, or a brain abscess might be suspected in a patient with these findings who also has a chronic ear, sinus, or lung infection. Isolation of anaerobic organisms from the cerebrospinal fluid is highly suggestive of parameningeal infection. Anaerobes may enter the cerebrospinal fluid via intraventricular leakage of a cerebral abscess, through extension of infection from a focus of osteomyelitis, or from an epidural abscess. Focal cerebral signs may be an indication of a space-occupying intracranial infection; they also may appear during the course of bacterial meningitis as a result of occlusive vascular injury. When focal cerebral signs develop, the history should be reviewed for any neurologic symptoms antedating the onset of the acute meningitis. Bacterial endocarditis may present with prominent symptoms of meningitis and a pleocytosis in the cerebrospinal fluid. This presentation is the result either of frank meningitis caused by pyogenic organisms or of sterile embolic cerebral infarctions produced by normally nonpyogenic organisms, such as Streptococcus viridans. Careful auscultation for cardiac murmurs and a search for peripheral signs of endocarditis (petechiae, splenomegaly, or Osler nodes) and echocardiography should be performed. Most patients with pneumococcal, meningococcal, or H. influenzae meningitis become afebrile within 2 to 5 days of the start of appropriate antibiotic therapy. Occasionally, fever continues beyond 8 to 10 days or recurs after having disappeared. Prolonged or recurrent fever accompanied by headache, focal cerebral signs, or obtundation suggests that antimicrobial therapy has been inadequate or a neurologic complication (e.g., cortical vein thrombophlebitis, ventriculitis, ventricular empyema, subdural effusion, or subdural empyema) has developed. Reevaluation of the findings in the cerebrospinal fluid, including gram-stained smears and cultures, is essential. Persistent fever in a patient whose clinical course and cerebrospinal fluid findings show progressive improvement may be indicative of drug fever.
General aspects of antibiotic treatment
Most antibiotics employed in the therapy of bacterial meningitis, with the exception of chloramphenicol, do not readily penetrate the noninflamed blood–brain barrier. Meningitis enhances the entry of penicillins and some other antimicrobial agents (e.g., vancomycin) into the cerebrospinal fluid and allows successful therapy with these drugs provided large parenteral doses are administered. Antibiotics should be administered intravenous in divided doses at intervals that provide high concentration gradients across the meninges. Dosage should not be decreased when clinical improvement occurs, because the normalization of the blood–brain barrier that accompanies resolution of the infection reduces antibiotic concentrations that can be obtained in the cerebrospinal fluid. The absence of intrinsic opsonic and bactericidal activity in infected cerebrospinal fluid increases the importance of providing bactericidal rather than bacteriostatic agents to treat bacterial meningitis. Although they are effective in vitro against many species that are capable of causing meningitis, drugs such as clindamycin, erythromycin, and first- and most second-generation cephalosporins (including cefamandole) should never be used in bacterial meningitis. These agents cannot predictably achieve bactericidal concentrations in the cerebrospinal fluid. Vancomycin, a microbiostatic agent, should also not be routinely used in the treatment of bacterial meningitis. It should be reserved for treatment of methicillin-resistant staphylococcal infections and for penicillin-resistant S. pneumoniae.
Warning
In the treatment of bacterial meningitis avoid:
- Drugs with poor penetration within the cerebrospinal fluid (e.g., clindamycin, erythromycin, first-generation cephalosporins, and most second-generation cephalosporins)
- Microbiostatic agents (e.g., vancomycin) unless no better alternative can be found.
Intrathecal therapy is not needed to treat uncomplicated cases of the three most common types of bacterial meningitis, since they can be treated with antimicrobials that enter the cerebrospinal fluid in bactericidal quantities. Only preservative-free gentamicin is available for intrathecal therapy.
Warning
Always check to make sure that the preparation you are using for intrathecal therapy is preservative free. If standard parenteral forms of aminoglycosides are used, a severe arachnoiditis often occurs. The patient’s clinical course dictates the frequency needed for examination of the cerebrospinal fluid. Repeat examination should be performed 24 to 48 hours after the start of antibiotic therapy if progress seems unsatisfactory or if the cause of the meningitis remains uncertain. Meningococcal meningitis should be treated until the patient remains afebrile for 5 to 7 days. With prompt and satisfactory response to antibiotics, it is not necessary to repeat the examination of the cerebrospinal fluid at the end of the therapy. Patients with H. influenzae meningitis should be treated for at least 7 days after they have become afebrile. Again, a follow-up examination of the cerebrospinal fluid is not necessary in patients who show rapid and complete clinical recovery. Patients with pneumococcal meningitis should be treated for 10 to 14 days. Prolonged therapy is necessary in the presence of an underlying mastoiditis or if the patient has underlying immunosuppression (e.g., neutrophil or B- or T-cell abnormalities or deficiencies).
Specific antimicrobial therapy
Bacterial meningitis is a life-threatening medical emergency that requires prompt therapy based on examination of the gram-stained smear from the sediment of the cerebrospinal fluid. Two serious but common errors in managing a patient with suspected meningitis are to delay performing a diagnostic lumbar puncture and to delay starting antibiotic therapy.
Warning
When imaging studies are needed to exclude an intracranial mass before a lumbar puncture is performed, antibiotics should be initiated immediately after blood has been obtained for culture. The choice of antibiotics depends at first on the suspected pathogen, and later on that which ultimately is isolated. The scan and the lumbar puncture may be done while the patient is receiving empirical antibiotic therapy. If a diagnostic lumbar puncture is performed after a mass lesion has been excluded by computerized tomography scan and the Gram stain reveals bacterial types not covered by the initial empirical therapy, the regimen can be appropriately altered. Animal models of meningitis suggest that initial empirical therapy will not affect the subsequent results of culture of cerebrospinal fluid if the spinal fluid is sampled within 2 to 3 hours of the start of antibiotic therapy.
| Table Initial Antibiotic and Anti-inflammatory Therapy for Suppurative Meningitis of Unknown Cause | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Clinical studies suggest that optimal chemotherapy for bacterial meningitis requires the cerebrospinal fluid concentration of the antibiotic to be several fold greater (>10 times) than the minimal microbicidal concentration for the pathogen measured in vitro. Additional principles that should guide therapy for meningitis include the following:
- The antibiotic must be capable of killing the pathogen.
- The pathogen must be shown to be highly susceptible to the selected antibiotic, as measured by quantitative dilution studies (e.g., in vitro cerebrospinal fluid killing levels or in vitro minimal microbicidal concentration tests).
- Because the antibiotic must reach local sites in concentrations sufficient to kill the pathogen, the agent selected must readily penetrate into the infected cerebrospinal fluid or, if not, be directly instilled into the cerebrospinal fluid by intrathecal or intraventricular injection.
- Foci of suppurative parameningeal infection must be drained whenever the procedure can be performed without causing serious neurologic damage. The extent to which various antibiotics transport into the cerebrospinal fluid during meningitis differs, and the practitioner must know this. In general, concentrations of antibiotic are higher in the cerebrospinal fluid of children and neonates than in adults with meningitis.
The presence of local leukocytes, especially neutrophils, is probably necessary for the infected meninges to become permeable to antibiotics. For example, limited experience suggests that vancomycin, which tends to accumulate in the cerebrospinal fluid of otherwise healthy adults with bacterial meningitis, does not enter the cerebrospinal fluid as well in neutropenic patients with documented bacterial meningitis. Therefore, serial determinations of the concentration of drug in the cerebrospinal fluid or serial studies of cerebrospinal fluid bacterial killing should be performed in severely neutropenic patients to document the presence of sufficiently high antibiotic concentrations. Otherwise, serial intrathecal or intraventricular injections of the appropriate antibiotics must be given. The third-generation cephalosporins usually achieve concentrations in cerebrospinal fluid that are at least 10 times the minimal microbicidal concentration against the common Enterobacteriaceae that cause meningitis (e.g., E. coli, Klebsiella species, and Proteus mirabilis). Such a concentration appears to be needed in order to cure meningitis. It is questionable, however, whether these agents, when used alone, can achieve 10-fold minimal microbicidal concentration concentrations in the cerebrospinal fluid against such organisms as Pseudomonas, Flavobacterium, Enterobacter, Serratia, and Acinetobacter species. The appearance of penicillinase-producing H. influenzae type b strains that are highly resistant to ampicillin (about 30% of isolates in the United States) has required a shift in the focus of initial management of this form of meningitis. Ceftriaxone or cefotaxime is now mandatory as empirical therapy for H. influenzae meningitis until the isolate has been demonstrated to be susceptible to ampicillin in vitro. Resistance to ampicillin in H. influenzae is caused by production of β-lactamase, whereas resistance to chloramphenicol is associated with production of acetyltransferase (Smith 1983). A patient with S. aureus meningitis should be treated with a penicillinase-resistant penicillin such as nafcillin or cloxacillin because 80 to 90% of S. aureus isolates are resistant to penicillin G. Enterococcal meningitis requires the use of intravenous penicillin or ampicillin supplemented by parenterally administered gentamicin. If a patient with enterococcal meningitis fails to respond promptly to parenteral therapy with penicillin and gentamicin, adjunctive intrathecal gentamicin (4 to 8 mg for an adult) may be given. The special problem of gram-negative bacillary meningitis The parenteral antibiotics that have been used in the past to treat gram-negative bacillary meningitis were ampicillin, chloramphenicol, and aminoglycosides. The results of treatment, however, were far from satisfactory: Mortality has ranged from 30% to 60%. The use of ampicillin as primary therapy for this form of meningitis is limited by the fact that about 30% of strains of gram-negative bacilli that cause neonatal meningitis, and the majority of isolates from adults with meningitis, are ampicillin-resistant. Chloramphenicol also has drawbacks in addition to its potential toxicity in neonates. Although the minimal microbicidal concentrations of chloramphenicol for many gram-negative bacilli (2 to 6 mg/L) are achievable in the cerebrospinal fluid, MBCs are generally so much higher (>60 mg/L) that they are not attainable in the cerebrospinal fluid. Adjunctive intrathecal antibiotic therapy in the treatment of gram-negative bacillary meningitis came into use for two reasons: 1) parenteral administration of gentamicin and tobramycin yields low (<1 mg/L) and inconsistent concentrations in the cerebrospinal fluid; and, 2) patients treated only with systemic agents have a high mortality. The high mortality associated with neonatal gram-negative bacillary meningitis, however, has not been reduced by the addition of lumbar intrathecal administration of gentamicin to parenteral therapy with ampicillin and gentamicin. Unfortunately, the ventricles are common sites of infection in bacterial meningitis. The unidirectional circulation of the cerebrospinal fluid inhibits drug entry into the ventricles, and little of the antibiotic introduced intrathecally in the lumbar area reaches the ventricular system. Adjunctive intraventricular administration of gentamicin either via a ventriculostomy reservoir or by percutaneous injection circumvents this obstacle. However, a controlled study of neonates with gram-negative bacillary meningitis demonstrated a higher mortality among infants who received intraventricular gentamicin along with systemic antibiotics (43%) than among those who received systemic antibiotics alone (13%). This finding suggests that intraventricular therapy with gentamicin is harmful to neonates with gram-negative bacillary meningitis. The adjunctive use of lumbar intrathecal aminoglycoside (e.g., gentamicin) was recommended in children and adults with gram-negative bacillary meningitis (other than H. influenzae meningitis) because of the high overall mortality associated with this disease when it was treated with parenteral antibiotics alone. The studies discussed above were performed before the development of the newer third-generation cephalosporins. A number of recent clinical trials support their efficacy in the treatment of patients in cases of meningitis that are caused by various bacterial agents and that occur in different age groups. Several general guidelines have been proposed regarding the use of the newer cephalosporins to treat bacterial meningitis:
- Final selection of the antibiotic or antibiotics to treat bacterial meningitis should be based on which drug or drugs have the greatest bactericidal activity for the causative agent, as determined by the minimal microbicidal concentration.
- The newer cephalosporins do not offer any advantage over penicillin G in the treatment of group B streptococcal meningitis.
- Cefotaxime, ceftriaxone, and ceftizoxime appear to be as effective as chloramphenicol or ampicillin in the treatment of H. influenzae meningitis;
- Cefotaxime and ceftriaxone appear to be equal to penicillin G or chloramphenicol to treat meningococcal and pneumococcal meningitides.
- Meningitis caused by Pseudomonas, Acinetobacter, Enterobacter, or Serratia cannot be successfully treated with third-generation cephalosporins alone.
- Meningitis caused by Listeria, staphylococci, or enterococci should not be treated with third-generation cephalosporins.
Warning
Third-generation cephalosporins lack activity against Listeria, staphylococci, and enterococci and are not adequate as monotherapy for many gram-negative bacilli.
Treatmen of bacterial meningitis of unknown cause
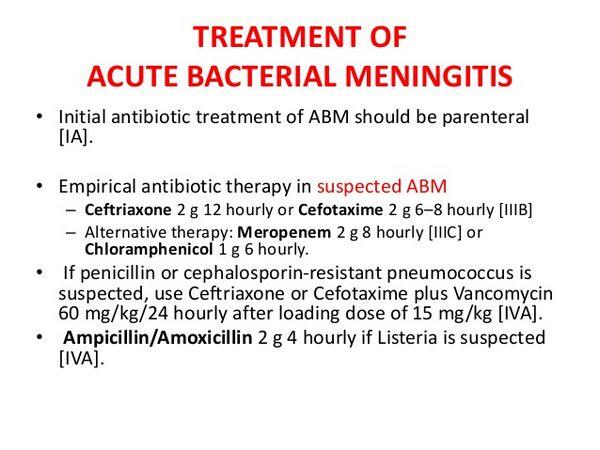
Empiric treatment of meningitis is directed at the most likely pathogens based on the age of the patient and available clinical clues. Meningitis in neonates may be caused by a wide range of enteric gram-negative bacilli and gram-positive organisms, such as E. coli, group B streptococci or Listeria. Such a variety of organisms necessitates the use of combined therapy with drugs such as ampicillin and either gentamicin or a third-generation cephalosporin such as cefotaxime. In children, antibacterial therapy is aimed at the three organisms most commonly responsible for childhood bacterial meningitis: S. pneumoniae, N. meningitidis, and H. influenzae with empiric ampicillin and ceftriaxone being most frequently employed. Streptococcus pneumoniae meningitis and N. meningitidis meningitis are the meningitides that most commonly affect adults, but the incidence of invasive H. influenzae infection in adults is on the rise. Listeria monocytogenes is the fourth most common cause of meningitis in adults. The combination of ampicillin and ceftriaxone is the treatment choice for bacterial meningitis of unknown cause in the adult because this combination is effective against S. pneumoniae, N. meningitidis, H. influenzae, and L. monocytogenes. The increase in high-level penicillin-resistant S. pneumoniae has led many authorities to recommend a combination of vancomycin and ceftriaxone for the empiric treatment of bacterial meningitis in communities where 2% or more of the isolates of S. pneumoniae exhibit high-level penicillin resistance. The incidence of uncommon types of meningitis in certain clinical settings has increased, such as meningitis caused by S. aureus in post craniotomy patients, and meningitis caused by gram-negative bacilli or Listeria in patients who are immunocompromised because of advanced age, neoplastic disease, or cirrhosis. Broader initial antibiotic therapy is warranted in the management of patients with these underlying conditions.
Adjunctive therapy of meningitis
Despite effective antibacterial therapy, morbidity and mortality from bacterial meningitis remain high. Data from animal studies suggest that modification of the inflammatory response should reduce these sequelae. A number of clinical trials in predominantly H. influenzae meningitis in infants and children demonstrated reduction in sensorineural deafness and neurological sequelae in the corticosteroid-treated group. There are limited data on the use of adjunctive dexamethasone therapy in adults with meningitis. A trial that included 429 Egyptian children and adults with bacterial meningitis involved randomization to antibiotics (ampicillin plus chloramphenicol) with or without adjunctive dexamethasone. The mortality from pneumococcal meningitis in the dexamethasone group was 13.5% (7/52) versus 40% (22/54) in the group without dexamethasone (P < 0.001). The dexamethasone group had no hearing loss (0/45) versus 12.5% (4/32) in the group with no steroid treatment (P < 0.05). A meta-analysis of 11 studies of corticosteroid adjunctive therapy in meningitis found that children receiving placebo were 3.77 times more likely to develop auditory dysfunction than those who received dexamethasone. McIntyre and colleagues also performed a meta-analysis of all trials of dexamethasone therapy in childhood meningitis published from 1988 to November 1996. They identified 16 studies, 11 of which fit their criteria for analysis. In H. influenzae meningitis, dexamethasone reduced severe hearing loss, odds ratio 0.31; (95% CI 0. 14–0.69). For all organisms combined, the pooled odds ratio suggested protection against neurologic deficits other than hearing loss but was not significant (odds ratio 0.59; 95% CI 0.34–1.02). In pneumococcal meningitis, only studies in which dexamethasone was given early suggested protection that was significant for severe hearing loss (odds ratio 0.09; 95% CI 0.0–0.71) and approached significance for any neurological or hearing deficit (odds ratio 0.23; 95% CI 0.04–1.05). Tunkel and Scheld (1996) recommend the use of dexamethasone in adults with meningitis if they have stupor or coma or cerebral edema documented by computerized tomography or magnetic resonance imaging, and/or evidence of mark elevated intracranial pressure. For maximum benefit the corticosteroid should be given before the antibiotics, since the antibiotics result in bacterial cell lysis and further inflammation.
| Table Adjunctive Therapy for Bacterial Meningitis | ||||||||||||
|
Chemoprophylaxis
Meningococcal meningitis is the only type of bacterial meningitis that occurs in epidemic form. Close contacts of an index case (such as other household members, infants in day-care centers, or military recruits) are at increased risk for developing meningococcal disease. Casual contacts such as schoolmates do not appear to be at increased risk. Hospital personnel in close patient contact (e.g., during nasotracheal suctioning or mouth-to-mouth resuscitation) are at increased risk, but personnel who come into contact with the patient after institution of respiratory precautions and antibiotic therapy are not. Chemoprophylaxis is indicated only for close contacts. Sulfonamides, once widely employed in chemoprophylaxis, should no longer be used for that purpose because approximately 25% of meningococcal isolates are now resistant to sulfonamides. Rifampin is the drug of choice to accomplish prophylaxis in close contacts. The recommended dose in adults is either 600 mg orally every 12 hours for 2 days or 600 mg orally once daily for 4 days. (Note the potential for enzyme induction, e.g., oral contraceptive.) About 50% of secondary cases among close contacts occur at least 5 days after onset of the disease in the index case patient. This fact prompts consideration of the use of meningococcal bivalent vaccine (groups A and C) as an adjunct to chemoprophylaxis, to extend protection should chemoprophylaxis be unsuccessful. Secondary cases of systemic H. influenzae type b infections may occur in close household and day-care center contacts of an initial case of H. influenzae meningitis. The risk for household contacts who are younger than 12 months is 6%; for those younger than 4 years, the risk is 2%. The risk of severe H. influenzae disease among household contacts appears to be 585 times greater than the age-adjusted risk in the general population. A variety of drugs, including ampicillin, trimethoprim-sulfamethoxazole, and cefaclor, have been tested but were ineffective in eradicating nasopharyngeal carriage of H. influenzae type b. Rifampin (20 mg/kg daily for 4 days) has been successful in eradicating carriage and appears to be the drug of choice for chemoprophylaxis. Chemoprophylaxis is given to household contacts (in households were there is a child younger than 48 months of age) of the index case. The use of chemoprophylaxis for day-care contacts is more controversial. In addition, active immunization of contacts with the polysaccharide vaccine of H. influenzae type b may be effective immunoprophylaxis against secondary cases of H. influenzae type b disease.


