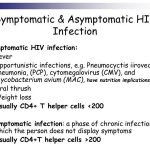
The same general principles of antiretroviral therapy that apply to HIV-infected adults also apply to HIV-infected pediatric patients; however, the treatment of HIV-infected neonates, children, and adolescents involves unique pharmacologic, virologic, and immunologic considerations. In 1993, the Working Group on Antiretroviral Therapy and Medical Management of HIV-infected Children, a panel convened by the National Pediatric and Family HIV Resource Center (NPHRC), the Health Resources and Services Administration (HRSA), and the National Institutes of Health (NIH) first issued guidelines for the use of antiretroviral agents in the treatment of HIV-infected children. At that time, monotherapy with zidovudine or didanosine was considered an appropriate regimen for initial therapy in HIV-infected pediatric patients.