In Africa, a wide variety of trypanosomes infect wild animals but only two cause significant disease in humans: T brucei gambiense and T brucei rhodesiense.
Essentials of Diagnosis
Epidemiologic factors: living or traveling in an endemic zone; exposure to tsetse fly.
History and physical exam:
- General: periodic fevers, wasting, nutritional deficiencies.
- Skin: chancre at the site of inoculation, fleeting truncal rash, posterior cervical lymphadenopathy.
- Neurologic: disturbed sleep patterns (diurnal somnolence, nocturnal insomnia), mental status changes, cerebellar signs.
Laboratory:
- Blood smear with Giemsa stain shows hemoflagellates.
- Aspiration and stain of chancre (may be positive for visible organisms before parasitemia occurs).
- Serology: indirect immunofluorescence, ELISA.
- Card agglutination test against common variant antigens.
- Cerebrospinal fluid (CSF): lymphocytic pleocytosis, elevated protein, motile trypanosomes.
General Considerations
Epidemiology
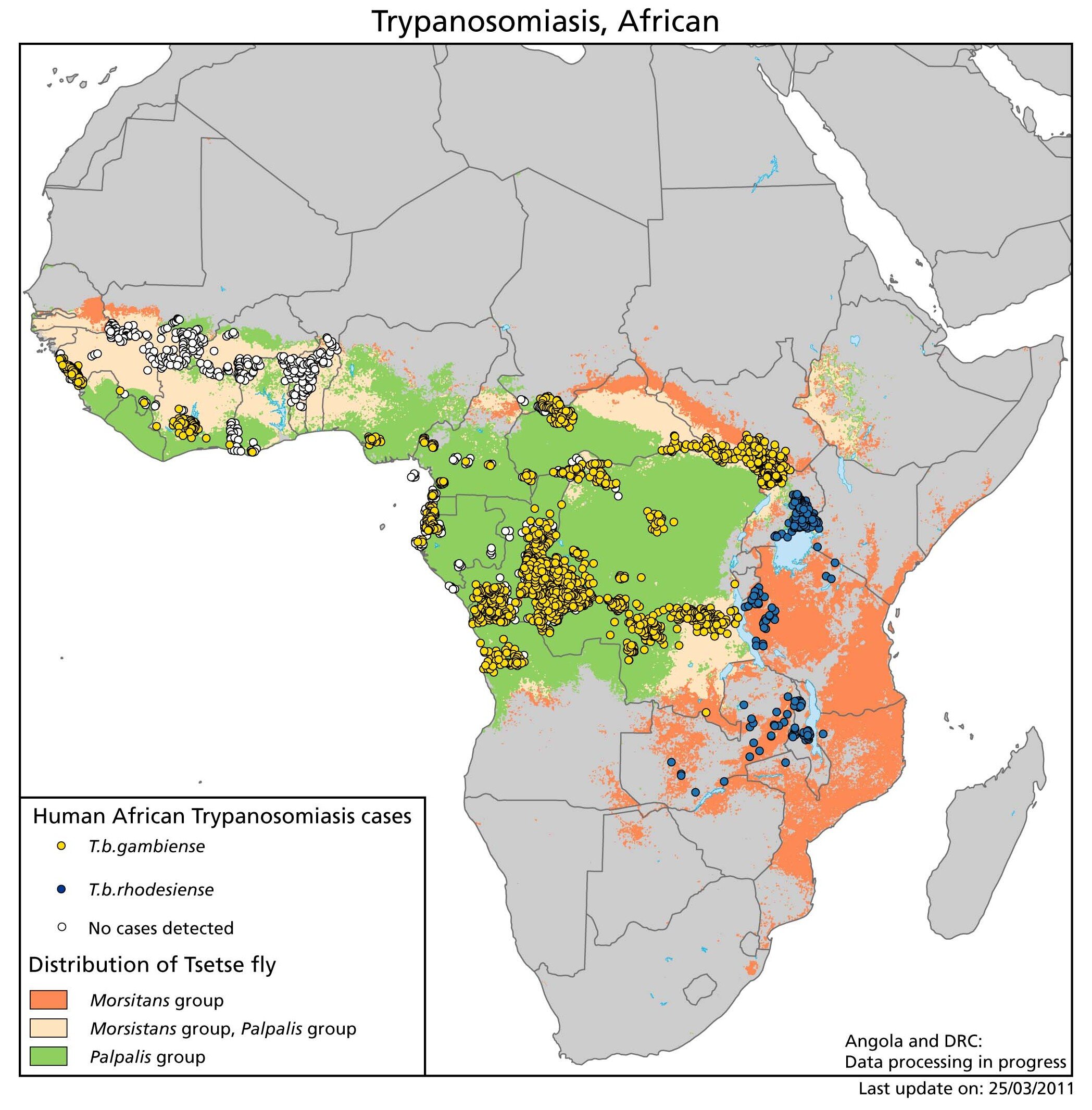
An estimated 50 million people are at risk for acquiring African trypanosomiasis worldwide, and there are 20,000 reported new cases annually. This is likely an underestimate because reporting in endemic countries is incomplete. There are no natural life cycles of T brucei outside Africa; thus, the only cases seen outside Africa are imported. Both T brucei rhodesiense and T brucei gambiense are carried by the tsetse fly vector of the genus Glossina, but by different species inhabiting distinct habitats. Therefore, because of vector habitat, T brucei rhodesiense (the agent of eastern African sleeping sickness) is seen in savanna and drier zones, whereas T brucei gambiense (the agent of western African sleeping sickness) is found near rivers and in forested areas. T brucei gambiense has primarily a human reservoir, while T brucei rhodesiense is an anthropozoonosis involving ungulates, such as cattle and antelope.
Transmission of T brucei gambiense was originally thought to be exclusively person to person, via insect vectors, but several animal hosts have been shown to harbor identical strains of the parasite, including pigs, cattle, dogs, sheep, and wild ungulates such as kob and hartebeest. The importance of these animal reservoirs remains uncertain. West African trypanosomiasis affects primarily rural populations, and the duration of the illness is months to years, which increases ongoing transmission. East African trypanosomiasis, in contrast, has a shorter clinical course, lasting < 9 months, and it primarily affects rural populations in proximity to the animal source and tourists visiting game parks. The animal reservoirs for T brucei rhodesiense include several domesticated animals, most importantly cattle, but a large number of wild animals including bushbuck, waterbuck, hartebeest, and lions. Many different domesticated animals become infected, but they succumb to the disease rapidly and are therefore unlikely to be important reservoirs for ongoing transmission. The number of infections in humans fluctuates tremendously depending on migration, land development programs, human conflict, and proximity of animal reservoirs to human populations. In addition to vector-borne transmission, congenital and blood transfusion transmission have been documented.
Microbiology
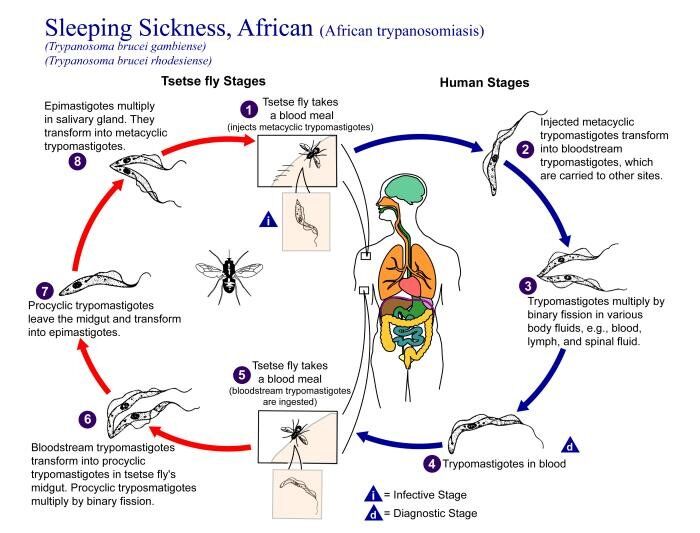
Blood-sucking tsetse flies of the genus Glossina become infective 18-35 days after taking a blood meal from an infected mammalian host. Trypomastigotes are ingested and multiply in the midgut of the fly. These migrate to the salivary glands and become epimastigotes, which then turn into short, stumpy infective metacyclic trypanosomes. The fly remains infective for life and transmits by subsequent bites. Both Glossina males and females feed on mammalian blood, causing infection if enough organisms are injected. In endemic areas, usually < 1% of flies are infected, whereas during epidemic periods as many as 5% of flies carry parasites.
Pathogenesis
During the course of the infection with trypanosomes, the number of parasites in the blood and lymph tissues fluctuates according to the host’s immune response. An increase in parasite number or parasitemia is related to the proliferation of parasite subpopulations that express an antigenically new or variant glycoprotein coat. A similar phenomenon is observed with Neisseria and Borrelia species. The declines in parasite number correspond with antibody-mediated destruction of trypanosomes. Each parasite carries genes encoding multiple, variant surface glycoproteins (VSG). Only one VSG is expressed at a single time, except during the switching from one VSG to another. This antigenic switching not only allows evasion of the host’s immune system but also poses a major challenge to the development of a vaccine. Acquired immunity does develop, but it is specific for a limited number of VSGs.
Resistance to African trypanosome infections depends on the presence of host interferon gamma and a strong Th1 cytokine response, as is the case for host resistance to Leishmania infections. Humoral and cellular immune responses are directed against the VSGs, among other trypanosomal components.
Clinical Findings
There are three stages of African trypanosomiasis: (1) an initial phase characterized by a skin chancre at the site of inoculation, (2) a blood-borne and lymphatic dissemination phase, and (3) invasion of the choroid plexus and the subarachnoid space, causing meningoencephalitis — hence the term “sleeping sickness.” Chancres are reported in about one-third of infections and generally appear on the exposed surface of the skin where the flies have bitten. A chancre lasts ~ 3 weeks. Initially chancres are warm and tender but then scar or infrequently ulcerate. Lymphadenopathy develops in the area draining the ulcer.
Once the ulcer subsides, the hemolymphatic stage appears, with characteristic periodic fevers coinciding with high parasitemia every day or two. Fatigue, arthralgia and headache, and often a fleeting truncal rash are observed with parasitemia. Patients are relatively asymptomatic between febrile periods. Myocarditis is common, and jaundice may occur from either hemolysis or hepatic damage.
In the meningoencephalitic stage, persistent headache, disturbed sleep patterns including nocturnal insomnia and diurnal somnolence, extrapyramidal and cerebellar signs, and behavioral changes are common features of disease. Wasting and nutritional deficiencies are common and may lead to secondary infection due to immunosuppression. The leukocyte count is normal or modestly elevated with a lymphocytosis. Polyclonal hypergammaglobulinemia, especially involving immunoglobulin M, is a striking and constant feature as might be expected from prolonged antigenic stimulation. Anemia and hypoalbuminemia are also observed.
Diagnosis
A Giemsa or Wright-stained smear of peripheral blood during the acute febrile period is the best means of obtaining a diagnosis. T brucei rhodesiense tends to have higher parasite loads and may be easier to detect than T brucei gambiense, which may require more frequent repeated blood smears or use of concentration techniques, eg, microhematocrit centrifugation with examination of the buffy coat. If a chancre is present, aspiration and staining may yield a diagnosis before parasitemia is present. Organisms may be demonstrated from aspirates of lymph nodes and bone marrow. The CSF should be examined even if parasitemia is confirmed. This should be done after clearance of the parasites from the blood so that parasites are not introduced into the CSF. In CNS trypanosomal infection, the CSF reveals a lymphocytic pleocytosis, elevated protein, and sometimes motile trypanosomes. Often morular or so-called Mott cells are seen, which are plasma cells with large eosinophilic inclusions containing immunoglobulin G. Several immunodiagnostic tests are available including indirect immunofluorescence, ELISA, and a card agglutination test that uses a commonly occurring variant antigen.
Treatment
The two forms of African sleeping sickness can be treated in a similar fashion. Eflornithine (difluoromethyl-ornithine) inhibits ornithine decarboxylase, an essential parasite enzyme, and is an effective drug in both the early and later CNS stages of disease (Box 5). The side effects include vomiting, abdominal pain, and diarrhea. Seizures occur in < 5% of cases. Unfortunately, there is a current shortfall in drug supply, with no signs of immediate improvement. Suramin has also been shown to be effective. It is available in the United States only through the CDC. The urine should be checked prior to each dose for protein, and the dose or interval between doses should be altered if the specimen is positive. Pentamidine has been used as an alternative agent, but it is not US Food and Drug Adminstration-approved for this indication. Drug information is available through the CDC (daytime phone: [404] 639-3670; nighttime, phone [404] 639-2888).
African sleeping sickness tends to relapse, even after appropriate therapy; in this event, treatment should be repeated. Because parasitic confirmation of relapse may not be possible, increasing CSF protein or pleocytosis may be used as a marker of relapse. Infection does not confer immunity, and reinfection presents as new infection, often with chancres. Despite treatment, this disease is fatal in ~ 7% of patients.
Drug used in the human African trypanosomiasis treatment
From the first decade of this century arsenicals have been the most universal and most effective drugs for all cases of sleeping sickness. Melarsoprol, introduced in the 1940s, remains the most universal of these compounds. However, resistance of trypanosomes and toxicity that may be fatal for the patient are two major shortcomings. Pentamidine, suramin and Berenil((R)) are active only in the first stage of the disease, when the parasites are confined to blood and lymph. Nifurtimox taken orally for 1 to 2 months and alpha-difluoro-methylornithine (alpha-DFMO) with an administration scheme spread over 5 weeks including 14 days of intravenous injections. provide interesting alternatives for all cases, since they reach the central nervous system. However, DFMO is known to be less active against. T. rhodesiense. Imidazoles, new arsenical derivatives and antimitotics have been successfully tested in experimental models. Combinations of drugs with additive or pontentiating effects mainly based on inhibition of decarboxylase enzymes or exposure to axidative stress appear promising.
Drug used in the treatment of sleeping sickness (human African trypanosomiasis: HAT)
Prevention & Control
One of the most effective public health measures for control of T brucei gambiense-associated disease may be recognition and treatment of humans, because they serve as the reservoir for this organism (Box 6). Control efforts that focus on clearance of tsetse fly habitat and destruction of wild game, as well as human population relocation, have not been terribly effective.
Individuals should avoid known foci of disease, wear protective clothing, and use insect repellents and mosquito nets to reduce the risk of infection. Chemoprophylaxis is not generally recommended for travelers to endemic areas. No vaccine is currently available.