Leishmania & Trypanosoma
The genera Leishmania and Trypanosoma are members of the family Trypanosomatidae. These protozoans cause diseases with widely varied clinical presentations as well as geographic distributions, including leishmaniasis, American trypanosomiasis (Chagas’ disease), and African trypanosomiasis (sleeping sickness). For example, the endemic zones for African and American trypanosomiasis do not overlap, the diseases are transmitted by different vectors, they involve distinct mechanisms of pathogenesis, and they follow different clinical courses. Nonetheless, the causative agents share important biological features. Each is a hemoflagellate with a kinetoplast containing its own chromosomal DNA with highly conserved and repeated elements, each forms a single flagellum at some point during its life cycle, and each is highly adapted to life within an insect.
Essentials of Diagnosis
- Epidemiologic factors: time spent in an endemic zone and exposure to sandfly vector.
- Physical exam: nonhealing ulcer (cutaneous infection); nonhealing mucosal membrane lesion, nasal obstruction, epistaxis, nasal septum perforation (mucocutaneous infection); fever, hepatosplenomegaly, emaciation (visceral infection).
- Laboratory examination of affected tissue:
- Giemsa stain shows phagosomal cells contain amatigotes at leading edge of biopsied ulcer.
- Histological analysis of bone, liver, or spleen tissue shows amastigotes.
- Culture of biopsied tissue, ie, skin, spleen, liver, or bone, reveals promastigotes.
- Serologic analysis using immunofluorescence assay (available through the Centers for Disease Control and Prevention [CDC]).
- Xenodiagnostic analyses of macerated tissue samples injected into animals for specific identification via growth in vivo.
- Polymerase chain reaction (PCR) (helpful but not widely available).
General Considerations
Infections with Leishmania spp. occur worldwide, in environments as varied as the semiarid deserts of the Middle East and the tropical rain forests of Central and South America. There are three major clinical forms of disease: visceral (kala-azar), mucocutaneous, and cutaneous. Even though each form tends to be associated with particular Leishmania species, some species can cause multiple forms of the disease, and some forms can be caused by multiple species. Leishmaniasis is a zoonosis with sandfly vectors and mammalian reservoirs. Lutzomyia sandflies are the primary vectors in the Americas, whereas Phlebotomine sandflies transmit the disease in the rest of the world.
Epidemiology
Leishmaniasis is endemic in = 82 countries, with an at-risk population of 350 million people worldwide. The World Health Organization (WHO) estimates 12 million current cases, with an annual incidence of 600,000 new cases and 75,000 deaths. Reliable estimates are scarce owing to lack of surveillance or active reporting and the large number of asymptomatic or subclinical cases, as well as the challenge of diagnostic confirmation.
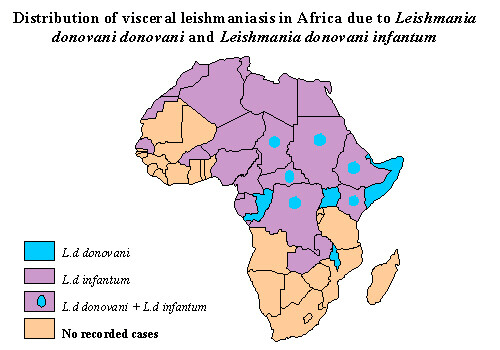
Leishmaniasis is endemic to all countries in the Americas except Canada, Uruguay, and Chile. Southern Texas is the only endemic area in the United States. There are often small geographic foci of transmission within endemic areas. Leishmaniasis is seen in the countries surrounding the Mediterranean Sea, as well as in central Africa and across the Middle East to India and China. Of visceral leishmaniasis cases worldwide, 90% occur in India, Bangladesh, southern Sudan, and northern Brazil; and 90% of cutaneous leishmaniasis cases occur in Afghanistan, Brazil, Iran, Saudi Arabia, and Syria. The vast majority of mucocutaneous leishmaniasis cases are found in Brazil and the geographic areas after which the subspecies and complexes are named (eg, amazonensis [Amazon region], panamensis [Panama], and guyanensis [Guyana]).
Leishmaniasis is most commonly observed in humans who live or work at the forest edge, where insect vectors are most abundant. These include rural settlers, farmers clearing forests, and road construction workers, as well as military personnel. Most cases of leishmaniasis in the United States are imported by those who have been exposed in rural areas of endemic regions, including Peace Corp workers, ornithologists, and field workers. Leishmaniasis is more common in adult males, probably in part because of occupational risks. Transmission of leishmaniasis has been associated with blood transfusions and intravenous drug abuse.
Although there are > 600 species of sandflies, only about one-tenth of these transmit leishmaniasis. Among these disease transmitters, both the Phlebotomine (found in the Mediterranean and Middle East regions) and the Lutzomyia (found in the Americas) sandflies are small and hairy and have a V-shaped wing configuration. They are inactive during daylight hours, and they stay in dark moist places that are rich in organic matter. The animal reservoirs in the Americas are primarily sylvatic and include sloths, opossums, and small forest rodents. Canines also serve as reservoirs for organisms that cause the visceral forms, especially as part of a peridomestic transmission cycle. With the notable exception of dogs and humans, the mammalian hosts that serve as reservoirs generally do not show signs of disease.
Microbiology and Pathogenesis
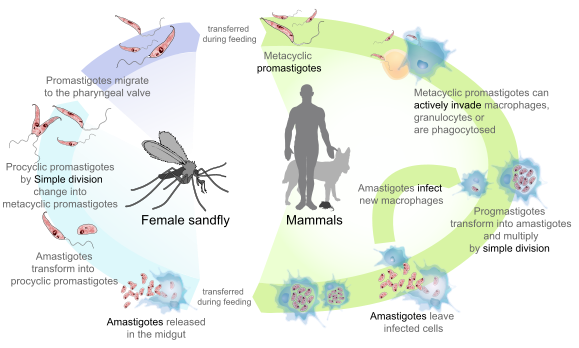
Species of the genus Leishmania display a dimorphic life cycle; in the insect vector they assume a flagellated form (the promastigote), and, in human and mammalian hosts, the parasite takes on the amastigote form (devoid of flagellum). Amastigotes survive inside phagosomal cells, such as the histiocytes of the skin and other cells of reticuloendothelial origin. Amastigotes are 2-3 um in length and can be round or oval. The species are morphologically indistinguishable from each other and from Trypanosoma cruzi, but Leishmania spp. can easily be differentiated from T cruzi in clinical specimens based on tissue tropism. The amastigotes transform into promastigotes once inside the sandfly gut. They then migrate forward to the proboscis to infect another host. Besides the insect vector, the life cycle usually includes a nonhuman animal reservoir. Humans are incidental hosts for Leishmania spp.
Promastigotes are passed from the female sandfly to the skin of a vertebrate host during a blood meal. These promastigotes invade the reticuloendothelial cells of the vertebrate host, change into amastigote forms, multiply further, and invade other reticuloendothelial cells. Mammalian blood is essential to the sandfly’s life cycle and promotes egg maturation within the female; only female sandflies seek blood meals. Once Leishmania amastigotes infect a sandfly, disease transmission to a new mammalian host is delayed for at least 7-10 days while the parasite undergoes differentiation.
The visceral form of leishmaniasis (kala-azar) provides an exception to this cycle from insects to animal reservoirs to humans, because there are no known animal reservoirs but only direct insect-to-human transmission. In India, for example, the organism and disease persist in the human population (anthroponoses) and are maintained by patients with persistent subclinical post-kala-azar dermal leishmaniasis, from whom parasites have been isolated from normal appearing skin.
Interferon gamma and other components of the Th1 cytokine response appear to be critical for host defense against disease. In an apparent attempt to subvert these defenses, Leishmania mexicana expresses several cysteine proteinases that down-regulate the Th1 cytokine response and induce the Th2 cytokines such as interleukin-4, thereby enhancing susceptibility of the host to disease progression.
Clinical Findings
Cutaneous Leishmaniasis
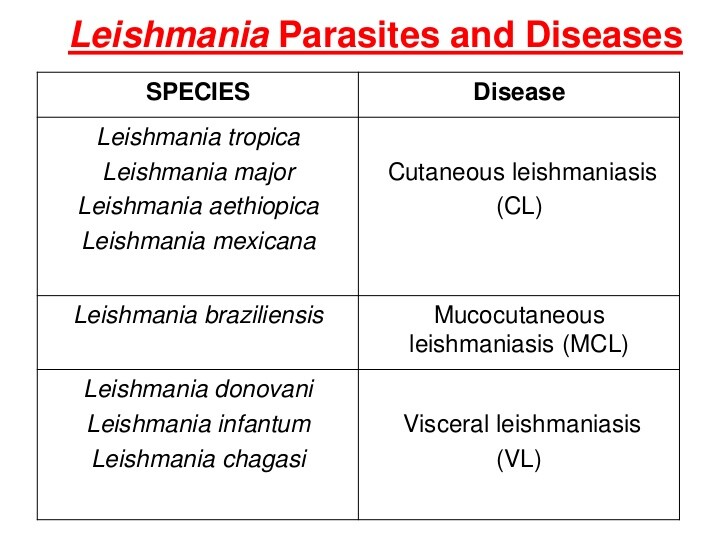
Cutaneous leishmaniasis begins as papules and, less commonly, as nodules at the site of the vector bite; it then progresses to ulcer formation. It is most commonly caused by Leishmania major and Leishmania tropica in the Old World and by Leishmania mexicana, Leishmania braziliensis, and L panamensis in the New World, and the infection exhibits an incubation period of 2-8 weeks. Satellite lesions appear in some cases. Most forms of the disease (> 80%) heal spontaneously within 3 months, although, on occasion, the cutaneous form can be disfiguring. In a more recently described form of cutaneous disease associated with L braziliensis, lymphadenopathy was found in three-fourths of patients and preceded the development of skin lesions; the latter healed spontaneously in only 20% of patients by 3 months after initial infection.
Mucocutaneous Leishmaniasis
Mucocutaneous leishmaniasis involves the mucosal membranes of the nose and mouth but may also involve the oropharynx and the larynx. L braziliensis is the cause of most mucocutaneous disease. An estimated 3% of patients with infection by this species develop mucosal disease. The disease begins as a primary cutaneous ulcer at the site of inoculation. During early stages it cannot be distinguished clinically from the more benign cutaneous forms of disease. The time delay from cutaneous disease to development of mucosal involvement can vary from 1 month to 24 years. Mucosal disease may present as nasal obstruction or epistaxis. A slight reddening and swelling in the septum can be seen, which may progress to perforation. Although the disease is termed mucocutaneous, tracheal and genital mucosal involvement is rare.
Visceral Leishmaniasis
Visceral leishmaniasis or kala-azar is primarily caused by Leishmania donovani in the Indian subcontinent and Africa, Leishmania infantum in the Mediterranean region, and Leishmania chagasi in Latin America. The incubation period is generally 2-6 months. The disease begins with fever, hepatosplenomegaly, and pancytopenia. The onset of fever can be sudden or gradual, and there may be intervals when the patient has low-grade or no fever. Clinically evident disease is usually characterized by progressive weakness, emaciation, and, if untreated, death. Case fatality rates, even with treatment, are = 11%. The ratio of inapparent infection to overt clinical disease varies depending on the age group and the endemic area but ranges from 6.5:1 to 18:1. This feature enables ongoing inapparent transmission of infection. A young age (infants and children) and malnutrition predispose to development of the disease. Viscerotropic (L tropica) disease in US troops who returned from the Persian Gulf was reported among a small number of individuals and resulted in a temporary ban on blood donations from this group.
The combination of visceral leishmaniasis and HIV infection is an increasingly important problem, especially in southern Europe. In general, the clinical presentation of visceral leishmaniasis in HIV-infected patients is similar to that in uninfected persons, but the gastrointestinal tract may be more frequently involved, and sometimes the hepatosplenomegaly is less pronounced or absent.
Diagnosis
The first step in diagnosis should be to establish an exposure history. It is important to distinguish the more innocuous cutaneous forms of leishmaniasis from the mucocutaneous forms, which are not usually self-limited. Therapy for leishmaniasis is not benign, and, therefore, diagnostic certainty is crucial. The differential diagnosis for each form of leishmaniasis is listed in Table 1.
The most common diagnostic approach is demonstration of the presence of parasites by microscopy in Giemsa-stained tissues or by culturing the organisms in specialized media. Aspiration and punch biopsy of the leading edge of a cutaneous or mucocutaneous lesion are the best techniques to obtain specimens for both culture and histology. The tissues of choice for diagnosis of visceral disease are liver, spleen, and bone marrow. Sternal bone biopsy is often the most easily accessible and safest procedure to obtain tissue for culture and smear. Giemsa-stained smears from either the culture (promastigotes) or the tissue biopsy specimens (amastigotes) show the distinctive rod-shaped kinetoplasts of Leishmania spp. in the cytoplasm of the parasite. The cytoplasm stains blue with Wright-Giemsa with a large eccentric red nucleus. Small red kinetoplasts are features of Leishmania spp. that distinguish them from other intracellular pathogens with somewhat similar appearance, such as Toxoplasma and Histoplasma spp.
Many diagnostic techniques, including DNA hybridization, PCR, and monoclonal antibody tests, have been developed. These tests are not widely available or standardized. Xenodiagnostic techniques are also used. Consultation with physicians experienced in the diagnosis of leishmaniasis is available through the CDC by calling (770) 488-7760.
Treatment
The drugs of choice for leishmaniasis include the organic antimonial compound stibogluconate sodium and meglumine antimoniate (Box 1). Anti-Leishmania drugs can be obtained from the CDC by calling (404) 639-3670.
Several alternative regimens and therapies are used, because of increasing resistance and treatment failures including higher dosing and, in some instances, combination therapy to decrease the duration of treatment. The newer drugs include liposomal amphotericin B, pentamidine, as well as paromomicin, and cytokines (eg, interferon gamma), which are used in combination with the antimonials.
Cutaneous Leishmaniasis
Cutaneous leishmaniasis is usually self-limited, but, if it persists > 6 months or is disfiguring or disabling, then treatment is indicated. The challenge for local therapy is that the disease characteristically involves the dermis and may disseminate to the lymph nodes or mucous membranes. Antimonials are injected with or without steroids initially, on a twice-daily schedule. Infiltration of the lesions in a standardized way is difficult to achieve. Localized controlled heat has also been shown, in a placebo-controlled clinical trial, to be effective for treatment of cutaneous disease.
Mucocutaneous Leishmaniasis
Mucocutaneous forms of leishmaniasis are treated with stibogluconate. The electrocardiogram (ECG) should be monitored. For resistant cases, stibogluconate plus interferon gamma can be used, or amphotericin B or pentamidine is effective.
Visceral Leishmaniasis
Visceral leishmaniasis (kala-azar) has successfully been treated with stibogluconate. In immunocompromised patients, such as those with HIV infection, it is necessary to devise a suppressive regimen to minimize recurrence after adequate treatment has been completed. Relapses of clinical disease typically occur after appropriate therapy with either antimony or amphotericin B (which has a higher initial cure rate in this population); therefore, a suppressive regimen has been suggested. Either pentamidine or paromomycin can be used alone as an acceptable choice for suppression, or either one can be combined with one of the following: interferon, ketoconazole, or fluconazole.
Prevention & Control
Because leishmaniasis is so widely endemic, control of both its reservoirs and its vectors remains a challenge (Box 2). Therefore, primary prevention efforts should focus on limiting vector contact, by using insect repellents and fine-mesh nets. No vaccine for humans is available. A strategy for preventing transmission of visceral leishmaniasis to humans in Latin America has been suggested, based on vaccination of dogs.
TRYPANOSOMA
Three human pathogens are members of the genus Trypanosoma. These are T cruzi, the agent of American trypanosomiasis (also known as Chagas’ disease), and T brucei subspecies rhodesiense and gambiense, both of which cause African sleeping sickness. Both of these diseases involve persistent circulation of parasites in the blood during some part of the disease course; these organisms are therefore referred to as hemoflagellates.
Trypanosomes have morphologically and physiologically different developmental stages in their insect and mammalian hosts. Like Leishmania spp., each stage contains a kinetoplast with highly conserved and repeating DNA sequences. Aside from morphology, the agents of African and American trypanosomiasis have little in common. The endemic zones of disease do not overlap, the organisms use different vectors, and their patterns of transmission are distinct. Furthermore, the clinical courses and response to therapy of these diseases are different.
American Trypanosomiasis (Chagas’ Disease)
Table 1. Differential Diagnosis of leishmaniasis.
Infections
Cutaneous
Mucocutaneous
Visceral
Bacterial
- Tularemia
- Furuncle/carbuncle
- Cellulitis
- Yaws
- Syphilis
- Atypical tuberculosis
- Tuberculosis
- Leprosy
- Tertiary yaws
- Syphilis
- Brucellosis
- Typhoid fever
- Tuberculosis
Fungal
- Histoplasmosis
- Sporotrichosis
- Lobomycosis
- Coccidioidomycosis
- Blastomycosis
- Chromomycosis
- Histoplasmosis
- Paracoccidiodomycosis
- Rhinosporidiosis
- Histoplasmosis
Viral
- 0Orf
- Infectious mononucleosis
Protozoan
- Drucunculiasis
- Acute Chagas’ disease
- Schistosomiasis
- Amebic liver abscess
- Malaria
Other (non-infectious)
- Basal or squamous cell carcinoma
- Lymphoma or metastatic cancer
- Discoid lupus
- Sarcoidosis
- Insect bite
- Ecthyma
- Kerion
- Pyogenic granuloma
- Foreign-body granuloma
- Basal cell carcinoma
- Wegener’s granulomatosis
- Mid-line granuloma
- Sarcoidosis
- Lymphoma
BOX 1. Treatment of Leishmaniasis
First Choice
Comments and Alternatives
Cutaneous
- Meglumine antimonate (glucantime), 20 mg/kg/d IM or IV for 10 d
- Stiboguconate, 20mg/kg/d IV or IM, preferably in two divided doses for 10 d
- Pentamadine, 2-4 mg/kg IM every other day × 7-15 injections
- Local treatment with intralesional injection of stibogluconate, every other day × 8-24 injections for nondisseminated infections with L major and L mexicana mexicana
Mucocutaneous
- Stibogluconate, 20 mg/kg/d IV or IM, preferably in two divided doses, × 30 d
Visceral
- Stibogluconate, 20 mg/kg/d IV or IM, preferably in two divided doses, × 30 d
- If resistance: pentamidine, 4 mg/kg 3 times week for 5 weeks
OR
- Amphotericin B, 1 mg/kg IV every other day × 20 d
- May reduce the course from 28 to 15 d by adding aminosidine (paromomycin), 15 mg/kg/d IM
BOX 2. Control of Leishmaniasis
Prophylactic
- Avoid exposure to sandfly vectors
Measures
- Chemoprophylaxis not recommended
- Immunoprophylaxis not available
Isolation Precautions
- None
BOX 3. Treatment of American Trypanosomiasis
Adult
Pediatric
First Choice
- Nifurtimox, 8-10 mg/kg/d PO in 4 divided doses for 120 d
- Nifurtimox, 15 mg/kg/d PO in 4 divided doses for 120 d
Alternative
- Benznidazole, 5 mg/kg/d PO for 60 d
- Benznidazole, 5 mg/kg/d PO for 60 d
BOX 4. Control of American Trypanosomiasis (Chagas’ Disease)
Prophylactic Measures
- Avoid exposure to reduviid insect vectors
- Chemoprophylaxis not recommended
- Immunoprophylaxis not available
Isolation Precautions
- None
BOX 5. Treatment of African Trypanosomiasis
Adult
Pediatric
First Choice
- Eflornithine, 400 mg/kg/d IM or IV in 4 divided doses for 14 d, then 300 mg/kg/d PO to complete 30 d
OR
- Suramin, 1 g IV on days 1, 3, 7, 14, 21; start with 200-mg test dose
- Suramin, 20 mg/kg IV on days 1, 3, 7, 14, 21; start with test dose
Alternative
- Pentamadine, 4 mg/kg/d IM × 10 d
- Pentamadine, 4 mg/kg/d IM × 10 d
BOX 6. Control of African Trypanosomiasis
Prophylactic Measures
- Avoid exposure to tsetse fly vectors
- Chemoprophylaxis not recommended
- Immunoprophylaxis not available
Isolation Precautions
- None