Itraconazole can be used to treat various superficial fungal infections, including dermatophytoses, pityriasis versicolor, and mucosal and cutaneous forms of candidosis. It is also effective in patients with subcutaneous infections, such as chromoblastomycosis, sporotrichosis, and certain forms of phaeohyphomycosis. It has become the drug of choice for non-life-threatening forms of blastomycosis and histoplasmosis and is an alternative to amphotericin B for invasive aspergillosis.
Drug Nomenclature
Therapeutic Classes of Itraconazole
Antifungal Agents, Antifungals, Antiprotozoal Agents, Antiprotozoals.
European Pharmacopoeia, 6th ed. (Itraconazole)
A white or almost white powder. Practically insoluble in water, slightly soluble in alcohol, freely soluble in dichloromethane, sparingly soluble in tetrahydrofuran. Protect from light.
Pharmacokinetics
Absorption of itraconazole from the gastrointestinal tract is incomplete (about 55%), but is improved if the drug is given with food. Oral administration of a single 100-mg capsule will produce peak serum concentrations of between 0.1 and 0.2mg/l about 2-4 h later. Higher concentrations are obtained after repeated dosing, but there is marked variation between individuals. As with ketoconazole, there is a disproportionate increase in blood levels with increasing dosage. Serum concentrations are markedly reduced when gastric acid production is impaired.
Much higher blood concentrations (up to 1.0-1.5 mg/1) have been attained in patients with AIDS and neutropenic individuals following administration of a 5 mg/kg dose of the oral solution formulation of itraconazole for 1-2 weeks. This formulation is better absorbed if given without food.
Like most other azole antifungals, the protein binding of itraconazole is high, exceeding 99% in human serum. As a result, concentrations of the drug in body fluids such as CSF are minimal. In contrast, drug concentrations in tissues, such as lung, liver, and bone, are two to three times higher than in serum. High concentrations of itraconazole are also found in the stratum corneum as a result of drug secretion in sebum. Itraconazole has been found to persist in the skin for 2-4 weeks after the end of a 4-week course of treatment. It persists in toenails for up to 6 months after the end of a 3-month course of treatment, but levels in the fingernails decline about 3 months after the end of treatment.
Less than 0.03% of an administered dose of itraconazole is excreted unchanged in the urine, but up to 18% is eliminated in feces as an unchanged drug.
Uses
IV and oral itraconazole capsules are used to treat systemic fungal infections in immunocompromised and immunocompetent patients. These include blastomycosis (pulmonary and extrapulmonary), histoplasmosis (including chronic cavitary pulmonary disease and disseminated nonmeningeal disease), and aspergillosis (pulmonary and extrapulmonary in patients who do not respond to or cannot tolerate amphotericin B).
Itraconazole oral solution (but not itraconazole capsules) is used for the treatment of oropharyngeal and esophageal candidiasis. Itraconazole (given IV initially followed by itraconazole oral solution) is used for empiric antifungal therapy in febrile neutropenic patients. Oral itraconazole capsules are used in immunocompetent individuals for the treatment of tinea unguium (onychomycosis) of the toenail and/or fingernail caused by dermatophytes.

Itraconazole is also used orally for the prevention of serious fungal infections (e.g., coccidioidomycosis, cryptococcosis, histoplasmosis, mucocutaneous candidiasis) in patients with human immunodeficiency virus (HIV) infection. Prior to initiation of IV itraconazole or oral itraconazole capsules for the treatment of systemic fungal infections, appropriate specimens for fungal culture and other relevant laboratory studies (wet mount, histopathology, serology) should be obtained in order to isolate and identify the causative organism(s). Itraconazole therapy may be started pending the results of these in vitro tests; however, once results are available, therapy should be adjusted accordingly. Prior to the initiation of oral itraconazole capsules for the treatment of onychomycosis, appropriate nail specimens for microbiological studies (e.g., potassium hydroxide [KOH] preparation, fungal culture, nail biopsy) should be obtained to confirm the diagnosis.
Aspergillosis
Itraconazole is used in the treatment of pulmonary and extrapulmonary aspergillosis in patients who are intolerant of or who are refractory to IV amphotericin B. IV amphotericin B is generally considered the treatment of choice for invasive aspergillosis, especially for life-threatening and severe infections, and itraconazole is an alternative agent. In a limited number of patients with invasive aspergillosis who did not respond to or could not tolerate IV amphotericin B, oral itraconazole capsules (200-400 mg daily given for a median duration of therapy of 3-4 months) have been effective as second-line therapy.
Blastomycosis
Itraconazole is used in the treatment of pulmonary and extrapulmonary blastomycosis caused by Blastomyces dermatitidis. While both oral itraconazole capsules and IV amphotericin B are considered drugs of choice for the treatment of blastomycosis, amphotericin B is preferred for the treatment of severe infections, especially those involving the CNS. IV amphotericin B is also generally preferred for the initial treatment of presumptive blastomycosis in immunocompromised patients, including HIV-infected individuals. Many clinicians consider oral itraconazole the drug of choice for the treatment of nonmeningeal, non-life-threatening blastomycosis, including mild to moderate disseminated infections without CNS involvement, and also recommend the drug for follow-up therapy in patients with more severe infections after an initial response has been obtained with IV amphotericin B.
Treatment failures have been reported when an oral antifungal agent (e.g., ketoconazole) was used to treat cutaneous or pulmonary blastomycosis in patients who had asymptomatic or subclinical CNS involvement at the time of the initial diagnosis. This should be considered when selecting an antifungal agent for blastomycosis patients. Some clinicians state that azole antifungal agents should not be used for the primary treatment of patients with CNS blastomycosis.
Histoplasmosis
Itraconazole is used in the treatment of histoplasmosis, including chronic cavitary pulmonary disease and disseminated nonmeningeal disease. Both IV amphotericin B and oral itraconazole capsules are considered drugs of choice for the treatment of histoplasmosis. However, IV amphotericin B is generally preferred for the initial treatment of severe, life-threatening histoplasmosis, especially in immunocompromised patients such as those with HIV infection.
Oral itraconazole is generally used in the initial treatment of mild to moderate infections (e.g., in patients who do not require hospitalization) or as follow-up therapy in the treatment of severe infections after a response has been obtained with amphotericin B. Itraconazole has been used for the treatment of disseminated histoplasmosis in patients with HIV infection.
The manufacturer states that data from a limited number of patients indicate that the response rate of histoplasmosis to itraconazole therapy in HIV-infected individuals is similar to that in patients not infected with this virus. However, the clinical course of histoplasmosis in HIV-infected individuals generally is more severe and usually requires long-term maintenance therapy to prevent relapse. Studies to further establish the safety and efficacy of the drug in the treatment of this infection in HIV-infected individuals, including investigation of optimum dosage and duration of maintenance therapy, are ongoing. Oral itraconazole is considered the drug of choice for primary prophylaxis and long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of histoplasmosis in HIV-infected individuals. (See Uses: Prevention of Fungal Infections in HIV-infected Individuals.)
Oropharyngeal and Esophageal Candidiasis
Because topical effects and drug exposure may be greater with the oral solution than with itraconazole capsules, only itraconazole oral solution should be used for the treatment of oropharyngeal and esophageal candidiasis. In 2 controlled studies in patients with oropharyngeal candidiasis (92% were HIV-infected), a clinical response (defined as cured or improved) was attained in 71-84% of patients receiving itraconazole oral solution. There is some evidence that itraconazole oral solution is at least as effective as oral fluconazole tablets and may be more effective than oral clotrimazole lozenges for the treatment of oropharyngeal candidiasis. Itraconazole oral solution has been effective for treating oropharyngeal candidiasis in some patients, including some HIV-infected individuals, who failed to respond to oral fluconazole.
Sporotrichosis
This medicine is used to treat sporotrichosis. While oral itraconazole may be effective in patients with mild to moderate pulmonary or disseminated sporotrichosis, IV amphotericin B is the drug of choice for the initial treatment of severe, life-threatening infections and whenever there is CNS involvement. Oral itraconazole is generally considered the drug of choice for the treatment of cutaneous, lymphocutaneous, or mild pulmonary or osteoarticular sporotrichosis and for follow-up therapy in more severe infections after a response has been obtained with IV amphotericin B.
Since sporotrichosis in immunocompromised patients (e.g., HIV-infected individuals) is particularly aggressive and difficult to treat, IV amphotericin B is probably the drug of choice for initial therapy in these patients; however, treatment failures occur. Some clinicians recommend that HIV-infected individuals who have been treated for sporotrichosis receive oral itraconazole for lifelong suppressive or maintenance therapy to prevent relapse; such prophylaxis is not addressed in current US Public Health Service and Infectious Diseases Society of America (USPHS/IDSA) guidelines for the prevention of opportunistic infections in individuals infected with HIV.
Onychomycosis
Oral itraconazole capsules are used in immunocompetent individuals for the treatment of onychomycosis of the toenails (with or without fingernail involvement) and onychomycosis of the fingernails caused by dermatophytes (tinea unguium).
Prior to administration of itraconazole capsules for the treatment of onychomycosis, appropriate nail specimens should be obtained for microbiologic studies (e.g., potassium hydroxide [KOH] preparation, fungal culture, nail biopsy) to confirm the diagnosis. In double-blind, placebo-controlled studies in patients with onychomycosis of the toenails, oral itraconazole (200 mg as capsules given once daily for 12 consecutive weeks) resulted in a mycologic cure in 54% of patients; 35% were considered an overall success (mycologic cure plus clear or minimal nail involvement with significantly decreased signs) and 14% had mycologic cure plus clinical cure (clearance of all signs, with or without residual nail deformity).
The mean time to overall success was approximately 10 months; however, 21% of those considered an overall success had a relapse of onychomycosis. In a double-blind, placebo-controlled study in patients with onychomycosis of the fingernails, oral itraconazole given in a pulse-dosing regimen (200 mg as capsules twice daily for the first week, no itraconazole during weeks 2-4, and 200 mg as capsules twice daily during the fifth week) resulted in a mycologic cure in 61% of patients; 56% were considered an overall success and 47% had mycologic cure plus clinical cure. The mean time to overall success was approximately 5 months; there were no relapses in those who were considered an overall success.
Paracoccidioidomycosis
Oral itraconazole capsules are used in the treatment of paracoccidioidomycosis (South American blastomycosis) caused by Paracoccidioides brasiliensis and are considered a drug of choice for the treatment of this infection. While the most effective regimen for the treatment of paracoccidioidomycosis in HIV-infected individuals has not been identified, some clinicians suggest that these patients receive initial therapy with IV amphotericin B, and that a less toxic agent (e.g., oral itraconazole capsules, co-trimoxazole) can then be used for long-term suppressive therapy for prophylaxis against recurrence or relapse; such prophylaxis is not addressed in current guidelines recommended by the USPHS/IDSA for the prevention of opportunistic infections in individuals infected with HIV.
Coccidioidomycosis and Cryptococcosis
Although not considered a drug of first choice, oral itraconazole capsules are used as an alternative agent for the treatment of coccidioidomycosis or cryptococcosis. Itraconazole capsules are also used as an alternative agent for primary prophylaxis of cryptococcosis or for suppressive or maintenance therapy to prevent recurrence or relapse of coccidioidomycosis or cryptococcosis in HIV-infected individuals.
Chromomycosis
Oral itraconazole capsules have been used with some success for the treatment of chromomycosis (chromoblastomycosis) caused by various dematiaceous fungi (e.g., Cladosporium, Exophiala, Fonsecaea, Phialophora).
Basidiobolomycosis
Oral itraconazole has been used in a limited number of patients to treat GI basidiobolomycosis, a zygomycosis caused by Basidiobolus ranarum. B. ranarum has been isolated worldwide from decaying vegetation and soil and from the GI tracts of reptiles, amphibians, fish, and insectivorous bats (including in the US). Basidiobolomycosis most commonly occurs in tropical and subtropical regions such as eastern and western Africa, and infection usually manifests as painless, subcutaneous nodules of the limbs, trunk, or buttocks secondary to traumatic inoculation.
GI infections are extremely rare and possibly the result of ingestion of contaminated soil (especially near rivers or lakes) or fruits or vegetables contaminated with soil or feces from infected reptiles or amphibians. From April 1994 through May 1999, 7 cases of GI basidiobolomycosis were identified in Arizona. Most cases of GI basidiobolomycosis have been successfully treated with oral itraconazole (400 mg daily given for 3-19 months) after partial surgical resection of the GI tract; however, it is unclear whether a clinical response would have been obtained if itraconazole had been used alone without surgical intervention. Although ketoconazole has also been reported to be effective in at least one patient, amphotericin B has been ineffective for the treatment of GI basidiobolomycosis in several patients.
Empiric Therapy in Febrile Neutropenic Patients
Itraconazole (given IV initially followed by itraconazole oral solution) is used for empiric therapy of presumed fungal infections in febrile neutropenic patients. The safety and efficacy of itraconazole for this indication have been evaluated in an open, randomized study in febrile neutropenic adults with hematologic malignancies; patients received either itraconazole (200 mg IV twice daily for 2 days, then 200 mg IV once daily from days 3-14 followed by itraconazole oral solution 200 mg twice daily to complete up to 28 days of therapy) or conventional IV amphotericin B (0.7-1 mg/kg daily for up to 28 days). The therapeutic success rate (defined as patient survival with resolution of fever and neutropenia within 28 days of therapy, absence of emergent fungal infections, use of study drug without premature discontinuance because of toxicity or lack of efficacy, and therapy for 3 or more days) was 47% for itraconazole and 38% for amphotericin B (intent-to-treat analysis). Although the overall response rate was higher in those receiving itraconazole, more patients receiving itraconazole discontinued the drug because of persistent fever or changed antifungal therapy because of fever and more patients receiving amphotericin B discontinued the drug because of intolerance.
Prevention of Fungal Infections in HIV-infected Individuals
Oral itraconazole is used in selected patients with HIV infection for primary prophylaxis against cryptococcosis or histoplasmosis and for long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of coccidioidomycosis, cryptococcosis, histoplasmosis, or mucocutaneous candidiasis.
The Prevention of Opportunistic Infections Working Group of the USPHS/IDSA has established guidelines for the prevention of opportunistic infections, including fungal infections, in HIV-infected individuals. These guidelines include recommendations concerning the prevention of exposure to opportunistic pathogens, the prevention of first disease episodes, and the prevention of disease recurrence. The USPHS/IDSA states that primary prophylaxis to prevent the first episodes of mucocutaneous candidiasis in HIV-infected adults, adolescents, infants, and children is not recommended.
While routine primary prophylaxis to prevent first episodes of coccidioidomycosis, cryptococcosis, or histoplasmosis in HIV-infected adults, adolescents, infants, and children is not recommended, the USPHS/IDSA states that primary prophylaxis against cryptococcosis or histoplasmosis may be considered in certain selected individuals. The USPHS/IDSA recommends that HIV-infected adults, adolescents, infants, and children who have completed initial therapy for documented coccidioidomycosis, cryptococcosis, or histoplasmosis receive long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of these fungal infections.
In addition, the USPHS/IDSA states that HIV-infected individuals who have frequent or severe recurrences of mucocutaneous candidiasis may benefit from long-term suppressive or maintenance therapy (secondary prophylaxis). Because of concerns regarding the use of oral azole antifungal agents during pregnancy, itraconazole should not be used for primary prophylaxis or for chronic suppressive or maintenance therapy in women who are pregnant. If a woman becomes pregnant while receiving itraconazole for prophylaxis and elects to continue the pregnancy, the drug should be discontinued.
Effective contraceptive measures are recommended for all HIV-infected women receiving an oral azole antifungal agent for suppressive therapy. Conventional IV amphotericin B may be the preferred agent if long-term suppressive or maintenance therapy against coccidioidomycosis, cryptococcosis, or histoplasmosis is indicated in an HIV-infected pregnant woman, especially during the first trimester.
Primary Prophylaxis
The safety and efficacy of oral itraconazole for primary prophylaxis of serious fungal infections in HIV-infected individuals has been evaluated in a prospective, randomized, placebo-controlled study in 149 patients with advanced HIV infection. There was failure of prophylaxis in 19% of those receiving oral itraconazole (200 mg once daily) and 29% of those receiving placebo. Prophylaxis failures related to invasive fungal infections (histoplasmosis, cryptococcosis, aspergillosis) were more frequent in those receiving placebo than in those receiving itraconazole; however, the incidence of prophylaxis failure due to recurrent or refractory mucosal candidiasis was similar in both groups. While itraconazole prophylaxis significantly delayed the time to onset of histoplasmosis and cryptococcosis, a survival benefit was not demonstrated in those receiving the drug.
Cryptococcosis
The USPHS/IDSA states that, although routine primary prophylaxis against cryptococcosis is not recommended, primary prophylaxis may be considered in HIV-infected adults and adolescents with CD4+ T-cell counts less than 50/mm3 and in infants and children with severe immunosuppression (as defined by age-adjusted criteria). Routine prophylaxis is not recommended because of the relative infrequency of cryptococcal disease, lack of evidence of survival benefit associated with prophylaxis, as well as other concerns (e.g., possibility of drug interactions, potential for development of resistance, cost considerations).
The need for primary prophylaxis or suppressive therapy against other fungal infections (e.g., coccidioidomycosis, histoplasmosis, mucocutaneous candidiasis) should be considered while making the decision concerning prophylaxis against cryptococcosis. Routine testing of asymptomatic individuals for serum cryptococcal antigen is not recommended because of the low probability that results will affect clinical decisions. HIV-infected individuals cannot completely avoid exposure to Cryptococcus neoformans; there is no evidence that exposure to pigeon droppings is associated with an increased risk for cryptococcosis. Oral fluconazole is the agent of choice for primary prophylaxis against cryptococcosis in HIV-infected adults, adolescents, infants, and children and oral itraconazole (given as capsules) is considered an alternative.
Histoplasmosis
The USPHS/IDSA states that primary prophylaxis against histoplasmosis may be considered in HIV-infected adults or adolescents with absolute helper/inducer (CD4+, T4+) T-cell counts less than 100/mm3 who are at especially high risk of exposure to Histoplasma capsulatum because of occupational exposure or who live in a community with a hyperendemic rate of histoplasmosis (at least 10 cases/100 patient-years) and also may be considered for HIV-infected infants or children with severe immunosuppression (as defined by age-adjusted criteria) who live in areas endemic for histoplasmosis.
When deciding whether to use primary prophylaxis against histoplasmosis in these HIV-infected individuals, clinicians should consider the local incidence of histoplasmosis, the possibility of drug interactions, toxicity, the development of resistance, cost, and the need for prophylaxis against other fungal infections (e.g., candidiasis, cryptococcosis).
Routine skin testing with histoplasmin or serologic testing for histoplasmosis antibodies or antigens in HIV-infected individuals who live in areas endemic for histoplasmosis is not predictive of disease and is not recommended. Although HIV-infected individuals living in or visiting histoplasmosis-endemic areas cannot completely avoid exposure to H. capsulatum, those with CD4+ T-cell counts less than 200/mm3 should avoid activities known to be associated with increased risk (e.g., creating dust while working with surface soil; cleaning chicken coops that are heavily contaminated with droppings; disturbing soil beneath bird-roosting sites; cleaning, remodeling, or demolishing old buildings; exploring caves). Oral itraconazole (given as capsules) is the agent of choice for primary prophylaxis against histoplasmosis in HIV-infected adults, adolescents, or pediatric patients; the USPHS/IDSA makes no recommendation regarding an alternative to itraconazole.
Prevention of Recurrence
Coccidioidomycosis: For long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse in HIV-infected adults, adolescents, infants, and children with documented coccidioidomycosis that has been adequately treated, the USPHS/IDSA recommends oral fluconazole as the drug of choice and IV amphotericin B or oral itraconazole (given as capsules) as alternatives. Long-term suppressive or maintenance therapy for prophylaxis against recurrence or relapse of coccidioidomycosis in HIV-infected individuals generally is continued for life. Although HIV-infected individuals receiving suppressive antifungal prophylaxis against coccidioidomycosis may be at low risk for recurrence of this fungal infection if their CD4+ T-cell count increases to greater than 100/mm3 while receiving potent combination antiretroviral agent therapy, the USPHS/IDSA states that data are insufficient to date to warrant a recommendation regarding discontinuance of prophylaxis in these individuals.
Cryptococcosis: For long-term suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse in HIV-infected adults, adolescents, infants, and children with documented cryptococcosis that has been adequately treated, the USPHS/IDSA recommends oral fluconazole as the drug of choice and IV amphotericin B or oral itraconazole (given as capsules) as alternatives.
Suppressive or maintenance therapy (secondary prophylaxis) to prevent recurrence or relapse of cryptococcosis in HIV-infected individuals generally is continued for life, unless immune recovery has occurred as a result of potent antiretroviral therapy.
Histoplasmosis: For long-term suppressive or maintenance therapy to prevent recurrence or relapse in HIV-infected adults, adolescents, infants, and children with documented histoplasmosis that has been adequately treated, the USPHS/IDSA recommends oral itraconazole (given as capsules) as the drug of choice and IV amphotericin B as an alternative. Long-term suppressive or maintenance therapy against histoplasmosis in HIV-infected individuals generally is continued for life. Although patients receiving suppressive antifungal prophylaxis may be at low risk for recurrence of histoplasmosis if their CD4+ T-cell counts increase to greater than 100/mm3 while receiving potent combination antiretroviral agent therapy, the USPHS/IDSA states that data are insufficient to date to warrant a recommendation regarding discontinuance of prophylaxis in these individuals.
Mucocutaneous Candidiasis: The USPHS/IDSA recommends long-term suppressive or maintenance therapy for adults and adolescents with a history of documented esophageal candidiasis (especially with multiple episodes), considering the potential for the development of resistant strains of Candida. In addition, the USPHS/IDSA recommends suppressive therapy for infants and children with severe, recurrent mucocutaneous candidiasis, especially those with esophageal candidiasis.
Although many experts do not recommend long-term suppressive therapy of recurrent oropharyngeal or vulvovaginal candidiasis, for the same reasons they do not recommend primary prophylaxis against candidiasis. The USPHS/IDSA states that suppressive therapy against candidiasis may be considered in HIV-infected individuals with frequent or severe recurrences of these infections.
However, several factors should be addressed when considering such therapy, including the impact of recurrences on the patient’s well-being and quality of life, the need for prophylaxis against other fungal infections, the cost of prophylaxis, drug toxicities, drug interactions, and the potential for development of drug resistance among Candida and other fungi. If long-term suppressive or maintenance therapy is indicated in HIV-infected adults, adolescents, infants, or children with frequent or severe recurrences of oropharyngeal, esophageal, or vaginal candidiasis, the USPHS/IDSA recommends oral fluconazole as the drug of choice and itraconazole (given as the oral solution) as an alternative.
Administration
Itraconazole is administered orally or by IV infusion.
Oral Administration
The bioavailability of oral itraconazole varies depending on whether the drug is administered as capsules or as an oral solution, and the manufacturer states that these preparations should not be used interchangeably. Itraconazole oral solution (not itraconazole capsules) is indicated for the treatment of oropharyngeal or esophageal candidiasis. While itraconazole oral solution should be administered without food if possible, itraconazole capsules should be administered with a full meal to ensure maximal absorption of the drug. The possibility that GI absorption of the drug may be decreased in patients with hypochlorhydria, which has been reported in HIV-infected individuals, should be considered.
IV Infusion
Commercially available itraconazole injection must be diluted prior to IV infusion. The entire contents of an ampul containing itraconazole injection (250 mg) should be added to the 0.9% sodium chloride injection diluent (50 mL) provided by the manufacturer to provide a solution containing 3.33 mg/mL in a resultant volume of 75 mL.
To ensure maximum safety and efficacy, the itraconazole solution must be prepared and administered correctly. The appropriate ratio of itraconazole to diluent must be used, and a final concentration of 3.33 mg/mL must be maintained to provide a stable admixture and avoid the formation of a precipitate.
Whenever the solution and container permit, Itraconazole injection and diluted solutions of the drug should be inspected visually for particulate matter and discoloration prior to administration. To provide a 200-mg dose of itraconazole, the diluted solution should be mixed gently, and then 15 mL should be withdrawn and discarded. The remaining 60 mL of diluted solution containing 3.33 mg/mL should be given by IV infusion over 60 minutes.
The infusion should be given using a controlled infusion device, the infusion set provided by the manufacturer, and a dedicated IV line. Itraconazole should not be mixed with other drugs and should not be administered through the same IV line as other drugs. Following completion of the infusion, the manufacturer recommends that the infusion set be flushed via the 2-way stopcock using 15-20 mL of 0.9% sodium chloride injection over 30 seconds to 15 minutes; the entire IV line should then be discarded. Bacteriostatic sodium chloride solution should not be used for the flush solution since the compatibility of itraconazole solution with flush solutions other than 0.9% sodium chloride is not known.
Dosage
Because of differences in oral bioavailability, itraconazole capsules and oral solution should not be used interchangeably on a mg-for-mg basis.
The dosage of itraconazole capsules should be based on the type and severity of infection, the identity of the causative organism, and the patient’s response to therapy. The drug appears to undergo saturable metabolism in the liver; therefore, increases in dosage can result in more than proportional increases in plasma concentrations.
For the treatment of life-threatening systemic fungal infections, IV itraconazole or oral itraconazole capsules should be initiated using a loading dosage. If IV itraconazole is used initially, the recommended loading dosage in adults is 200 mg IV twice daily for 4 consecutive doses followed by 200 mg once daily thereafter.
Although clinical studies evaluating the safety and efficacy of oral itraconazole capsules did not include a loading dosage, based on pharmacokinetic considerations, the manufacturer and some clinicians state that oral itraconazole capsules should be initiated in life-threatening infections in adults using an initial loading dose of 200 mg three times daily (600 mg daily) for the first three to four days of therapy. Subsequent therapy can then be continued at the usual oral dosage of 200-400 mg daily.
The manufacturer states that itraconazole therapy should be continued for at least three months until clinical parameters and laboratory tests indicate that the active fungal infection has subsided. Some clinicians state that while the optimal duration of therapy for serious fungal infections has not been established, itraconazole therapy should probably be continued for at least 12 months for disseminated or chronic pulmonary histoplasmosis and for 6-12 months for blastomycosis. An inadequate period of treatment can result in the recurrence of the active infection.
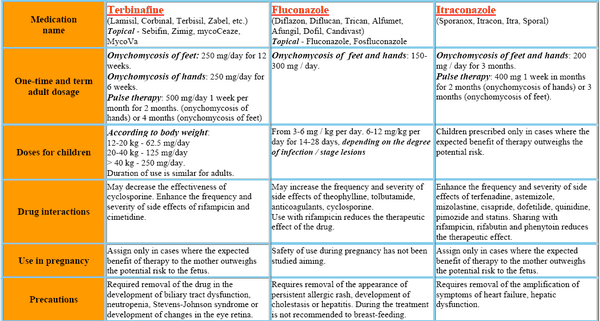
| Condition | Dosage | Administration Route | Duration |
|---|---|---|---|
| Pulmonary/Extrapulmonary Aspergillosis | 200-400 mg daily; higher doses (up to 600 mg) may be used for invasive cases | Oral/IV | IV: 200 mg twice daily for 4 doses, then 200 mg once daily; Oral: as needed |
| Blastomycosis/Histoplasmosis | Initial: 200 mg once daily; increase by 100 mg increments to max 400 mg daily | Oral/IV | At least 6 months for blastomycosis; 12-24 months for chronic histoplasmosis |
| Oropharyngeal Candidiasis | 100 mg/day for non-compromised; 200-400 mg/day for neutropenic patients | Oral solution | Minimum of 3 weeks |
| Esophageal Candidiasis | 100 mg (10 mL) daily, may increase to 200 mg (20 mL) daily based on response | Oral solution | Minimum of 3 weeks |
| Vaginal Candidosis | Two doses of 200 mg taken 6-8 hours apart | Oral | Single day treatment |
| Sporotrichosis | 100-200 mg once daily for cutaneous infections; 200 mg twice daily for osteoarticular | Oral | 3-6 months for cutaneous; 12 months for osteoarticular |
| Onychomycosis | 200 mg once daily for toenails (12 weeks); pulse dosing of 400 mg once daily for a week each month for 3 months | Oral | Varies by regimen |
| Empiric Therapy in Febrile Neutropenic Patients | IV: 200 mg twice daily for 4 doses, then decrease to 200 mg once daily; oral: 200 mg (20 mL) twice daily until recovery | IV/Oral | Up to 14 days IV, then oral until recovery |
| Cutaneous Dermatophytoses | 100 mg/day | Oral | Tinea corporis/cruris: 2 weeks; Tinea pedis/manuum: 4 weeks |
| Pityriasis Versicolor | 200 mg/day | Oral | 1 week |
| Subcutaneous/Deep Fungal Infection | 200-400 mg/day; loading doses of up to 600 mg/day may be used for life-threatening infections | Oral | Ongoing as needed |
| Long-term Maintenance Therapy in AIDS Patients | Histoplasmosis/Cryptococcosis: 200 mg/day; Neutropenic patients: 400 mg/day | Oral |
Generally continued for life
|
Prevention of Fungal Infections in HIV-infected Individuals
| Condition | Dosage | Administration Route | Duration |
|---|---|---|---|
| Primary Prophylaxis against Cryptococcosis | Adults/Adolescents: 200 mg once daily; Infants/Children: 2-5 mg/kg every 12-24 hours | Oral | Ongoing as needed |
| Primary Prophylaxis against Histoplasmosis | Adults/Adolescents: 200 mg once daily; Infants/Children: 2-5 mg/kg every 12-24 hours | Oral | Ongoing as needed |
| Secondary Prophylaxis for Coccidioidomycosis | Adults/Adolescents: 200 mg twice daily; Infants/Children: 2-5 mg/kg every 12-48 hours | Oral | Generally continued for life |
| Secondary Prophylaxis for Histoplasmosis | Adults/Adolescents: 200 mg twice daily; Infants/Children: 2-5 mg/kg every 12-48 hours | Oral | Generally continued for life |
| Secondary Prophylaxis for Cryptococcosis | Adults/Adolescents: 200 mg once daily; Infants/Children: 2-5 mg/kg every 12-24 hours | Oral | Generally continued for life |
| Secondary Prophylaxis for Mucocutaneous Candidiasis | Adults/Adolescents: 200 mg once daily; Infants/Children: 5 mg/kg once daily | Oral solution | Generally continued for life |
Dosage in Renal and Hepatic Impairment
Adjusting oral itraconazole dosage in patients with renal impairment does not appear necessary. Itraconazole injection should not be administered to patients with renal impairment (i.e., creatinine clearance less than 30 mL/minute) since severe renal impairment reduces the clearance of hydroxypropyl-b-cyclodextrin (an excipient in itraconazole injection).
While the effect of hepatic impairment on itraconazole pharmacokinetics currently remains to be elucidated, the manufacturer states that plasma concentrations of the drug should be monitored carefully in patients with such impairment.
In the UK, itraconazole oral liquid is licensed for use in oral and oesophageal candidiasis at a dose of 200 mg daily for one week. It may be taken as a single daily dose or, preferably, in two divided doses, with the liquid retained in the mouth for 20 seconds before swallowing. If there is no response after a week, treatment may be continued for another week.
In the USA, a similar regimen is licensed for oropharyngeal candidiasis, but for oesophageal candidiasis, an alternative regimen of 100 mg daily for at least three weeks is preferred, although the dose may be increased to 200 mg daily if necessary.
Some authorities in the USA, such as the Centers for Disease Control and Prevention, recommend higher dose. Itraconazole may also be given by intravenous infusion at a dose of 200 mg twice daily over 1 hour for two days, then 200 mg daily thereafter.
High Doses
Doses of itraconazole 600 mg daily in two divided doses for 3 to 16 months were used in 8 patients with systemic mycoses resistant to conventional therapy. Two patients with AIDS and cryptococcal meningitis failed to respond, and 2 who responded initially later relapsed or developed progressive disease when the dose was reduced. The main adverse effects were hypokalaemia, hypertension, and edema, possibly associated with adrenal suppression. In a patient with cerebral aspergillosis, itraconazole 800 mg daily for 5 months and then 400 mg daily for a further 4 / months produced a complete resolution of cerebral lesions.
Administration in Children
| Indication | Age Group | Dosage | Duration |
|---|---|---|---|
| Tinea Capitis | Children < 20 kg | 50 mg daily by mouth | As prescribed |
| Children ≥ 20 kg | 100 mg daily by mouth | As prescribed | |
| Oropharyngeal Candidiasis | 1 month to 12 years | 3 to 5 mg/kg (max 100 mg) daily | 15 days |
| 12 to 18 years | 100 mg (200 mg for neutropenia or AIDS) daily | 15 days | |
| Dermatophyte Infections | 1 month to 12 years | 3 to 5 mg/kg daily (max 200 mg for pityriasis versicolor, max 100 mg for tinea corporis, cruris, pedis, manuum) | Varies (7-30 days, depending on infection type) |
| 12 to 18 years | 200 mg daily for pityriasis versicolor; 100 mg daily for tinea corporis and cruris; 100 mg daily or 200 mg twice daily for tinea pedis and manuum | Varies (7-30 days, depending on infection type) | |
| Onychomycosis | 1 to 12 years | Courses of 5 mg/kg daily for fingernails (2 courses) and toenails (3 courses) | Repeated after intervals of 21 days |
| 12 to 18 years | Either 200 mg daily for 3 months or courses of 200 mg twice daily for fingernails (2 courses) and toenails (3 courses) | Repeated after intervals of 21 days | |
| Histoplasmosis/Systemic Fungal Infections | 1 month to 18 years | 5 mg/kg (max 200 mg) once or twice daily | As prescribed; twice daily for invasive disease |
| Prophylaxis in Neutropenia | 1 month to 18 years | 5 mg/kg (max 200 mg) daily, increased to twice daily if plasma itraconazole concentrations are low | As prescribed |
| Prophylaxis in Hematological Malignancy | 1 month to 18 years | 2.5 mg/kg twice daily | Start before transplantation or chemotherapy |
| Intravenous Doses for Systemic Infections | 1 month to 18 years | 2.5 mg/kg (max 200 mg) every 12 hours for the first two days, then once daily for a maximum of 12 days | As prescribed |
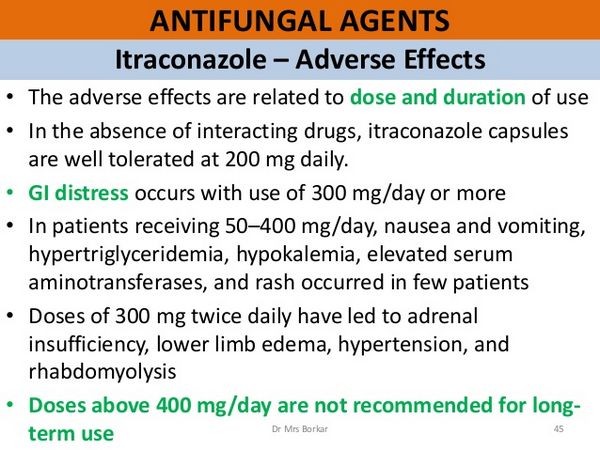
Side Effects

The most common adverse effects associated with itraconazole include dyspepsia, abdominal pain, nausea, vomiting, constipation, diarrhea, headache, and dizziness. Others include allergic reactions such as pruritus, rash, urticaria, and angioedema. Isolated cases of the Stevens-Johnson syndrome have been associated with itraconazole. An increase in liver enzyme values has occurred in some patients, and cases of hepatitis and cholestatic jaundice have been observed, especially in those treated for more than one month.
There have been rare cases of liver failure and death. Heart failure and pulmonary edema have been reported rarely, and serious cardiovascular events, including arrhythmias and sudden death, have been attributed to drug interactions in patients receiving itraconazole (see Interactions below). Alopecia, edema, and hypokalaemia have also been associated with prolonged use.
Menstrual disorders and peripheral neuropathy have been reported in a few patients.
Incidence of Adverse Effects
Itraconazole 50 to 400 mg daily for a median of 5 months was considered to be well tolerated in 189 patients with systemic fungal infections. of 86 patients with underlying disease, including 49 with AIDS, 16 with diabetes, and 23 with malignancy, nausea and vomiting occurred in 19 patients, hypertriglyceridaemia in 16, hypokalaemia in 11, and elevated liver enzyme values in 13. The role of itraconazole in hypertriglyceridemia could not be assessed because all the samples were not drawn in the fasting state, and hypertriglyceridemia is a complication of HIV infection.
Gynaecomastia occurred in 2 patients, 1 of whom also took spironolactone.
Rash occurred in 4 patients. Of 49 patients taking itraconazole 100 to 400 mg daily for up to 39 months, 23 did not experience adverse effects during treatment, while 6 had nausea and vomiting, 5 developed edema, and 2 developed hypertension 3 of the patients who developed edema and 1 who became hypertensive were diabetic. Three patients stopped itraconazole, 1 due to vomiting, 1 to leucopenia, and 1 to nephrotic syndrome. The patient with the nephrotic syndrome had pre-existing edema and hypertension. The syndrome cleared when itraconazole was stopped.
Cardiovascular
Ventricular fibrillation has been attributed to itraconazole-induced hypokalemia.
Pleural and subsequent pericardial effusion developed in a woman treated with itraconazole 200 mg bd for a localized pulmonary infection with Aspergillus fumigatus. After more than 9 weeks of treatment, she developed a pericardial effusion, which necessitated drainage. Itraconazole was withdrawn. Six weeks later, and 2 weeks after the resumption of itraconazole, she developed signs of pulmonary edema and cardiac enlargement. These signs disappeared rapidly on discontinuation of itraconazole.
Studies in dogs and healthy human volunteers have suggested that itraconazole has a negative inotropic effect; the mechanism is unknown. A systematic analysis of data from the FDA’s Adverse Event Reporting System (AERS) identified 58 cases suggestive of congestive heart failure in patients taking itraconazole. A simultaneous search did not identify any cases of congestive heart failure in patients taking fluconazole and ketoconazole, ruling out the possibility of a class effect. In consequence, the labeling of itraconazole has been revised. Itraconazole is now contraindicated for the treatment of onychomycosis in patients with evidence of ventricular dysfunction.
For systemic fungal infections, the risks and benefits of itraconazole should be reassessed if signs or symptoms of congestive heart failure develop.
Nervous System
Headache due to itraconazole has been mentioned in some reports. Dizziness is an uncommon complaint, as are mood disturbances.
Psychological, Psychiatric
Visual hallucinations with confusion have been reported in a 75-year-old woman, occurring on three separate occasions, each time about 2 hours after a 200 mg dose of itraconazole. Her symptoms abated spontaneously over about 8 hours.
Electrolyte Balance
Hypokalemia, occurring either in isolation or with hypertension, has been reported regularly in a small fraction of patients. Marked ankle edema with weight gain was seen in a patient taking itraconazole 400 mg/day, for whom there was no explanation other than the use of the drug; after withdrawal of the itraconazole, the symptoms disappeared. Hypokalemia and edema have also been observed in a number of patients taking high-dose therapy (600 mg/day), associated with mildly depressed aldosterone concentrations.
Gastrointestinal
Dyspepsia, pyrosis, nausea, vomiting, mild epigastric discomfort, and diarrhea can occur in patients taking itraconazole. These gastrointestinal complaints are generally mild, but they seem to be the most frequent adverse effects during treatment. The total incidence of adverse effects was 3-5% in patients treated for superficial mycosis and 8% in 99 patients treated for deep mycosis. A multicenter trial reported an incidence closer to 15%.
In 50 women with acute vaginal candidiasis, adverse effects were reported in 17 (35%), nausea in seven, headache in six, dizziness in three, and bloating in three, while aspartate transaminase activity was raised in one.
Of 1108 patients with HIV treated for mucosal candidiasis, 239 reported gastrointestinal symptoms.
Pseudomembranous colitis has been reported in association with exposure to itraconazole.
A 54-year-old man developed new abdominal pain and non-bloody diarrhea 1 month after exposure to a 7-day course of oral itraconazole 200 mg/day. He was taking stable chronic sertraline, valproic acid, and perphenazine and had not taken antimicrobial drugs for 6 months. Flexible sigmoidoscopy after clinical progression showed pseudomembranes, and subsequent evaluation excluded other causes of diarrhea. Although Clostridium difficile culture and toxin assay were eventually negative, possibly because of delayed stool sampling, he responded to a 10-day course of anti-anaerobic drug therapy and was discharged with completely resolved symptoms.
The authors proposed that itraconazole had disrupted the resident fungal flora of the colon.
Liver
In most clinical reports, there were some cases of raised liver enzyme activities; the changes were transient or disappeared after the withdrawal of itraconazole. More serious hepatotoxicity was not reported.
Focal nodular hyperplasia of the liver has been reported in a 38-year-old woman who had taken itraconazole 200 mg/day for 4 months for a fungal infection of the fingernails. She had taken no other drugs in the year during which focal nodular hyperplasia developed.
Of three patients, two women aged 62 and 57 and a man aged 75 years, who developed symptomatic hepatic injury 5-6 weeks after starting to take itraconazole, two had the biochemical pattern of cholestatic liver damage.
All itraconazole clinical trials sponsored by the Janssen Research Foundation for the treatment of onychomycosis, in which there was an assessment of laboratory safety, have been analyzed. There were no significant differences in the number of code 4 abnormalities (baseline value is in the reference range and at least two values, or the last testing in the observation period, exceed twice the upper limit of the reference range) in liver function tests (alanine transaminase, aspartate transaminase, alkaline phosphatase, and total bilirubin). The incidence of all the code 4 abnormalities was under 2%. Itraconazole pulse therapy for onychomycosis appears to be safe, especially from the perspective of potential liver damage. In the itraconazole package insert, liver function tests are recommended in patients receiving continuous itraconazole for over 1 month. There is no such monitoring requirement for the pulse regimen, unless the patient has a history of underlying hepatic disease, the liver function tests are abnormal at baseline, or signs or symptoms suggestive of liver dysfunction develop at any time.
Skin
Patients taking itraconazole have reported different types of rash, including acneiform rash. In one case, there were bloody bullae.
A 29-year-old man developed an infiltrative maculopapular eruption after 1 week of itraconazole 100 mg bd for tinea corporis. Itraconazole was withdrawn, and the lesions disappeared within 7 days. Scratch tests, patch tests, scratch-patch tests, and drug-induced lymphocyte stimulation tests for itraconazole were negative; however, rechallenge with systemic itraconazole induced a maculopapular eruption on the face, hands, and the dorsal of the feet. Empty itraconazole capsules had no cutaneous effects, suggesting an allergic reaction to a metabolite of the compound.
Photosensitivity has been attributed to itraconazole (200 mg qds for 5 days), with a reduced minimal erythema dose for both UVB (0.12 J/cm) and UVA (20.1 J/cm), negative photo patch testing, and a positive photo challenge. The authors proposed a photoallergic mechanism because earlier exposure to itraconazole had been uneventful. However, details about sun exposure during the first exposure and the intensity of sun exposure during the oral photo challenge procedure were not given. The eruption responded to oral steroids, which is more typical of photoallergic than phototoxic reactions.
The risk of serious skin disorders has been estimated in 61 858 users of oral antifungal drugs, aged 20-79 years, identified in the UK General Practice Research Database. They had received at least one prescription for oral fluconazole, griseofulvin, itraconazole, ketoconazole, or terbinafine. The background rate of serious cutaneous adverse reactions (corresponding to non-use of oral antifungal drugs) was 3.9 per 10 000 person-years (95% CI = 2.9). Incidence rates for current use were 15 per 10 000 person-years (1 for itraconazole, 11.1 (3 for terbinafine, 10 (1 for fluconazole, and 4.6 (0 for griseofulvin. Cutaneous disorders associated with the use of oral antifungal drugs in this study were all mild.
Sexual Function
There are inconsistent reports about the effects of itraconazole on sex steroids. Concentrations of testosterone, corticosterone, and progesterone remained unchanged in rats and six dogs in whom possible endocrine effects were studied. On the other hand, the administration of itraconazole to seven male volunteers for two weeks did not produce detectable changes in plasma testosterone or cortisol concentrations. Two weeks after the start of high-dose itraconazole therapy (600 mg/ day), one of eight patients with severe mycosis showed a slightly reduced cortisol response to ACTH stimulation.
Erectile impotence, with normal steroid concentrations, has been reported, as has a reduction in libido.
Immunologic
Itraconazole 200 mg bd for 2 weeks caused a serum sickness-like reaction in a 53-year-old woman with Meniere’s disease.
Most of the reported adverse effects of itraconazole are transient. Gastrointestinal reactions, mild dyspepsia, pyrosis, nausea, vomiting, diarrhea, and epigastric pain are not uncommon. In many of the published reports mention is made of increases in serum liver enzyme activities and hypertriglyceridemia, and symptomatic liver toxicity has been reported. Itraconazole does not induce drug-metabolizing enzymes and is a weaker inhibitor of microsomal enzymes than ketoconazole. In rats given doses of up to 160 mg/kg, there was no induction or inhibition of the metabolism of xenobiotics.
Hypokalemia has often been reported without an explanation of the mechanism. Higher doses (400 or even 600 mg/day) cause an increased incidence of adverse effects; among those documented at these dosages are severe hypokalemia, reversible adrenal insufficiency, and (in one published case) arrhythmias, the latter connected with an interaction with terfenadine. Skin rashes and pruritus have been reported. Tumor-inducing effects have not been described.
The most common adverse effects in patients taking itraconazole capsules for prolonged periods were nausea and vomiting (under 10%), hypertriglyceridemia (9%), hypokalemia (6%), raised transaminases (5%), rashes and/or pruritus (2%), headache or dizziness (under 2%), and foot edema (1%).
In a study using the UK General Practice Research Database to determine rates of rare, serious drug-induced, adverse effects on the liver, kidneys, skin, or blood, occurring within 45 days of completing a prescription or refill in 54 803 users of either fluconazole or itraconazole, one patient had an abnormal liver function test while taking itraconazole in whom a drug-induced etiology could not be ruled out, a rate of 3.2 per 100 000 prescriptions (95% CI for serious adverse liver effects. Thus, itraconazole does not commonly have serious adverse effects on the liver, kidneys, skin, or blood.
Interactions
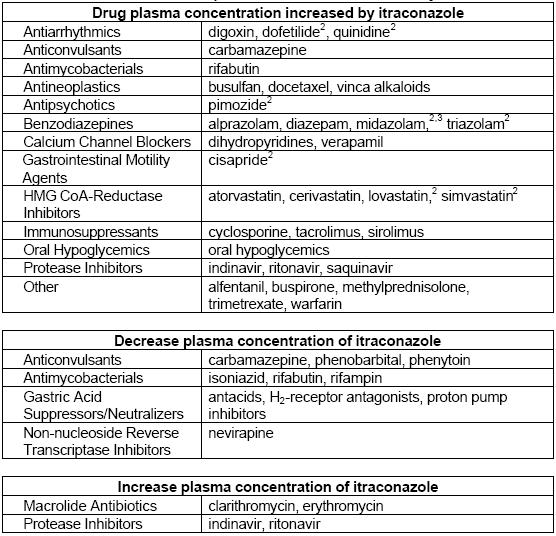
| Category | Medicine | Interaction with Itraconazole | Effects |
|---|---|---|---|
| Statins | Atorvastatin | Increased serum concentrations due to CYP3A4 inhibition. | AUC increased by 150%, Cmax increased by 38%, half-life prolonged by 30%. |
| Cerivastatin | Modest increase in serum concentrations; secondary CYP2C8 pathway unaffected by itraconazole. | Small increases in AUC, Cmax, and half-life (up to 51%, 25%, and 23% respectively). | |
| Fluvastatin | No significant effect on Cmax or AUC; slight prolongation of half-life. | Minimal interaction; considered safe with itraconazole. | |
| Lovastatin | Greatly increased plasma concentrations even at low doses of itraconazole. | Cmax increased ~15-fold, total AUC increased >15-fold; caution advised. | |
| Pravastatin | Slight increases in AUC and Cmax; changes not statistically significant. | Half-life remains unchanged; minimal interaction observed. | |
| Simvastatin | Markedly increased systemic exposure when combined with itraconazole. | Cmax and AUC increased at least 10-fold; significant risk of rhabdomyolysis noted in some cases. | |
| Glucocorticoids | Budesonide | Increased metabolic clearance inhibition leading to adrenal failure in some patients. | AUC increased 4.2-fold, half-life prolonged from 1.6 to 6.2 hours; significant cortisol suppression observed. |
| Dexamethasone | Markedly increased systemic exposure and effects due to reduced clearance by itraconazole. | AUC increased nearly four-fold, half-life prolonged more than three-fold; lower cortisol levels noted. | |
| Methylprednisolone | Significant increase in exposure due to CYP3A4 inhibition by itraconazole. | AUC increased 3.9-fold, half-life prolonged from 2.1 to 4.8 hours; enhanced adrenal suppression observed. | |
| Prednisolone | Minor interaction with limited clinical significance compared to methylprednisolone. | AUC increased by 24%, half-life extended by 29%; generally safe to use with itraconazole. |
Other Interactions
Tacrolimus
Tacrolimus concentrations and toxicity are affected by itraconazole.
- In a 17-year-old man with cystic fibrosis who received a hepato-pulmonary transplant, there was an interaction of itraconazole 600 mg bd with tacrolimus. High trough concentrations of tacrolimus were noted, despite the relatively low dosage (0.1-0.3 mg/kg/day).
- Another patient experienced an interaction of tacrolimus 0.085 mg /kg bd with itraconazole 200-400 mg per day, with resulting ketoacidosis, neutropenia, and thrombocytopenia, requiring the withdrawal of both drugs.
- A 30-year-old man with a renal transplant had a more than two-fold increase in blood tacrolimus concentrations after starting to take itraconazole 200 mg/day, accompanied by a reduced glomerular filtration rate and biopsy-proven tacrolimus-associated tubulopathy.
Because tacrolimus has a narrow therapeutic index, blood concentrations should be monitored particularly carefully when itraconazole is co-administered, and the dosage of tacrolimus may have to be altered.
The interaction of itraconazole (100 mg bd) with tacrolimus has been studied in 28 heart or lung transplant recipients. Tacrolimus blood concentrations were monitored on alternate days for up to 21 days after the start of itraconazole therapy or withdrawal. The dose of tacrolimus was adjusted to keep the 12-hour trough blood concentration at 7-12 micrograms/ml.
The mean dose of tacrolimus during itraconazole therapy fell significantly from 8.4 to 2.9 mg/ day. There was no significant change in serum creatinine or liver function tests. In patients in whom itraconazole was withdrawn, the mean dose of tacrolimus required increased significantly from 4.7 to 8.8 mg/day. Thus, substantial changes in the dose of tacrolimus were required both when itraconazole was begun and when it was withdrawn, and it was difficult to maintain tacrolimus blood concentrations within the target range during the first 2 weeks. However, major toxicity or rejection did not occur.
Co-administration of itraconazole may reduce the cost of post-transplant immunosuppression. This interaction is probably due to itraconazole’s inhibition of CYP3A4.
Vinca Alkaloids
Enhanced and potentially life-threatening neurotoxicity of vinca alkaloids through concomitant therapy with itraconazole has been the subject of several compelling reports. Enhancement of vincristine neurotoxicity results in polyneuropathy and paralytic ileus. The interaction is reversible, and the readings-traction of vinca alkaloids may be safe after a prolonged washout. The mechanism has not been formally elucidated but may be either competition for oxidative metabolism, leading to increased systemic exposure, or inhibition of the transmembrane P glycoprotein efflux pump, leading to increased intracellular concentrations of vinca alkaloids. The concomitant use of itraconazole and vinca alkaloids is therefore contraindicated.
Two adults with acute lymphoblastic leukemia developed unusually severe neurotoxicity caused by vincristine, which was probably the result of an interaction with itraconazole suspension.
Warfarin
Itraconazole can alter warfarin concentrations.
Following the addition of itraconazole to a treatment regimen comprising warfarin, ranitidine, and terfenadine, cardiac dysrhythmias developed in a 62-year-old man. The signs and symptoms included prolongation of the QT interval and ventricular fibrillation.
This particular regimen apparently resulted in a second interaction since unexpectedly high concentrations of terfenadine were found. Others have described this phenomenon, which involves a marked rise in terfenadine serum concentrations and increased drug toxicity during concurrent ingestion of itraconazole. The mechanism is not known, but it is likely related to inhibition of CYP3A4.
Zolpidem
Zolpidem is mainly transformed by CYP3A4. However, itraconazole 200 mg did not alter the pharmacokinetics and pharmacodynamics of zolpidem 10 mg in 10 healthy volunteers. Therefore, zolpidem may be used in normal or nearly normal doses together with itraconazole.
Amphotericin
Six strains of A. fumigates were used to test the in vitro effects of the combination of amphotericin with itraconazole. After pretreatment, an antagonistic effect was found for all strains in vitro and for one strain in a mouse model of aspergillosis.
The effect of the combination of itraconazole with amphotericin on liver enzyme activities has been studied retrospectively in 20 patients with hematological malignancies or chronic lung disease complicated by fungal infection or colonization. They took itraconazole 200-600 mg/day for a median of 143 (range 44 days. Nine had no abnormal liver function tests, including periods of high concentrations of itraconazole (over 5000 µg/ml) and its active hydroxylated metabolite; only one had received concomitant amphotericin. All of the 11 patients with liver function abnormalities had received concomitant amphotericin.
For each patient, liver function abnormalities were greatest during concomitant therapy with both antifungal drugs. Although liver enzyme abnormalities are uncommon with amphotericin, and although this retrospective analysis was subject to several flaws and potential biases, it nevertheless suggests that hepatotoxicity should be carefully monitored if itraconazole and amphotericin are co-administered.
Antihistamines
It seems likely that combining itraconazole with astemizole and terfenadine will lead to increased effects of these antihistamines.
Barbiturates
Barbiturates lower itraconazole concentrations.
Benzodiazepines
The effect of itraconazole on the single oral dose pharmacokinetics and pharmacodynamics of estazolam has been studied in a double-blind, randomized, crossover study in 10 healthy male volunteers, who took oral itraconazole 100 mg/day or placebo for 7 days and on day 4 a single oral dose of estazolam 4 mg. Blood samplings and evaluation of psychomotor function by the Digit Symbol Substitution Test, Visual Analogue Scale, and Stanford Sleepiness Scale were conducted up to 72 hours after estazolam. There was no significant difference between the placebo and itraconazole phases in peak plasma concentration, clearance, and half-life. Similarly, psycho-motor function was unaffected. These findings suggest that CYP3A4 is not involved to a major extent in the metabolism of estazolam.
In a study of the effects of itraconazole 200 mg/day and rifampicin 600 mg/day on the pharmacokinetics and pharmacodynamics of oral midazolam 7.5-15 mg during and 4 days after the end of the treatment, switching from inhibition to induction of metabolism caused an up to 400-fold change in the AUC of oral midazolam.
Bupivacaine
The interaction of itraconazole 200 mg orally od for 4 days with a single intravenous dose of racemic bupivacaine (0.3 mg /kg given over 60 minutes) has been examined in a placebo-controlled crossover study in 10 healthy volunteers. Itraconazole reduced the clearance of R-bupivacaine by 21% and that of 5-bupivacaine by 25%, but had no other significant effects on the pharmacokinetics of the enantiomers. Reduction of bupivacaine clearance by itraconazole is likely to increase steady-state concentrations of bupivacaine enantiomers by 20-25%, and this should be taken into account in the concomitant use of itraconazole and bupivacaine.
Buspirone
The interaction of itraconazole with the active l-(2-pyrimidinyl)-piperazine metabolite of buspirone has been studied after a single oral dose of buspirone 10 mg. Itraconazole reduced the mean AUC of the metabolite by 50% and the Cmax by 57%, whereas the mean AUC and Cmax of the parent drug were increased by 14.5-fold and 10.5-fold, respectively. Thus, itraconazole caused relatively minor changes in the plasma concentrations of the active piperazine metabolite of buspirone, although it had major effects on the concentrations of buspirone after a single oral dose.
Busulfan
Reduced elimination and increased toxicity of busulfan co-administered with itraconazole have been informed.
Carbamazepine
Low and sometimes very low serum concentrations of itraconazole have been seen during concurrent therapy of itraconazole with carbamazepine.
Ciclosporin
The combination of itraconazole with ciclosporin leads to a marked increase in blood ciclosporin concentrations, which can raise serum creatinine. This clearly points to renal damage caused by the high concentrations of ciclosporin. However, an interaction has not been demonstrated in all cases.
Two cases of rhabdomyolysis caused by itraconazole in heart transplant recipients taking long-term ciclosporin and simvastatin have been reported. To avoid severe myopathy, ciclosporin concentrations should be monitored frequently, and statins should be withdrawn, or the dosage should be reduced, as long as azoles need to be prescribed in transplant recipients. Patients need to be educated about signs and symptoms that require immediate physician intervention.
Citrate-phosphate Buffer
The citrate-phosphate buffer used to facilitate the absorption of dideoxyinosine (didanosine), prescribed for the treatment of AIDS, may interfere with the absorption of itraconazole.
Clarithromycin
A report of three HIV-negative patients has suggested that concomitant therapy with itraconazole and clarithromycin can lead to increased clarithromycin exposure, with an increased metabolic ratio, possibly related to itraconazole’s effect on CYP3A4. Nevertheless, in none of the three reported individuals were there adverse effects from this presumed interaction.
Clozapine
Itraconazole 200 mg had no significant effect on serum concentrations of clozapine 200-550 mg/day or desmethylclozapine in 7 schizophrenic patients.
Digoxin
Itraconazole inhibits the elimination of digoxin, eventually leading to toxicity.
Itraconazole increases the digoxin AUC0_72 by about 50%, and reduces its renal clearance by about 20%. Apart from inhibition of the renal secretion of digoxin, which is probably mediated by inhibition of P glycoprotein, a study in guinea pigs also showed significantly reduced biliary excretion of digoxin by itraconazole, suggesting that the interaction between itraconazole and digoxin may not only be due to a reduction in renal clearance, but also to a reduction in the metabolic clearance of digoxin by itraconazole.
The importance of this interaction has been emphasized by a report of two renal transplant patients who had digoxin toxicity when they took itraconazole concurrently.
Famotidine
Famotidine 40 mg/kg/day reduced the peak and trough concentrations of itraconazole 200 mg/kg/day by about 35% in 18 patients undergoing chemotherapy for hematological malignancies.
Fentanyl
Fentanyl is a substrate of CYP3A4, CYP2C9, and CYP2C19. However, in one study, the pharmacokinetics and pharmacodynamics of fentanyl 3 micrograms/kg were similar after itraconazole 200 mg and placebo in 10 healthy volunteers.
An interaction of itraconazole with fentanyl has been reported in a 67-year-old man with cancer on a stable dose of transdermal fentanyl 50 micrograms/hour. He took itraconazole 200 mg bd for oropharyngeal candidiasis and, 24 hours later, developed signs of opioid toxicity, which was reversed by withdrawal of fentanyl and replacement with short-acting opioids.
This may be an interaction to which only some individuals are susceptible.
Flucytosine
Combining itraconazole with flucytosine can enhance its activity against black fungi; this combination has prevented the development of flucytosine resistance.
Important Safety Information
Age
The safety, tolerability, and pharmacokinetics of itraconazole and its active metabolite hydroxyitraconazole after administration of itraconazole solution in hydroxy-propyl-P-cyclodextrin have been investigated in a multi-center study in 26 infants and children aged 6 months to 12 years with mucosal candidiasis or at risk of invasive fungal disease. There was a trend to lower minimum plasma concentrations in children aged 6 months to 2 years. The systemic absorption of the solubilizer hydro-xypropyl-P-cyclodextrin was less than 1%. Given at 5 mg/ kg/day, this formulation provided potentially therapeutic concentrations in plasma, somewhat lower than those attained in adults, and it was well tolerated and safe.
Itraconazole 100 mg/day has been studied in 24 children with Trichophyton tonsurans tinea capitis. Itraconazole was well tolerated, but 15 children required re-treatment due to persistent infection.
The safety, pharmacokinetics, and pharmacodynamics of an oral suspension of cyclodextrin itraconazole (2.5 mg /kg od or bd for 15 days) have been investigated in an open, sequential, dose-escalation study in 26 children and adolescents, 5-18 years old, infected with HIV (mean CD4 count 128 x 106/1) with oropharyngeal candidiasis. Apart from mild to moderate gastrointestinal disturbances in three patients, cyclodextrin itraconazole was well tolerated.
Two patients withdrew prematurely because of adverse events. The oropharyngeal candidiasis score fell significantly from a mean of 7.46 at baseline to 2.8 at the end of therapy, demonstrating antifungal efficacy in this setting. Based on these results, a dosage of 2.5 mg/ kg bd was recommended for the treatment of oropharyngeal candidiasis in children aged 5 years and over.
The safety and efficacy of oral cyclodextrin itraconazole (5 mg/kg/day) as antifungal prophylaxis has been assessed in an open trial in 103 neutropenic children (median age 5 years; range 0-15 years). Prophylaxis was started at least 7 days before the onset of neutropenia and continued until neutrophil recovery. Of the 103 patients, only 47 completed the course of prophylaxis; 27 withdrew because of poor compliance, 19 because of adverse events, and 10 for other reasons. Serious adverse events (other than death) occurred in 21 patients, including convulsions, suspected drug interactions, abdominal pain, and constipation. The most common adverse events considered definitely or possibly related to itraconazole were vomiting, abnormal liver function, and abdominal pain.
The study medication’s tolerability at the endpoint was rated as good (55%), moderate (11%), poor (17%), or unacceptable (17%). There were no unexpected problems with safety or tolerability.
Pregnancy, Fertility and Lactation
Although there are no adequate and controlled studies in humans to date, itraconazole has been shown to be teratogenic and embryotoxic in animals. Therefore, itraconazole should be used during pregnancy only when the potential benefits justify the possible risks to the fetus.
For the treatment of onychomycosis, the use of itraconazole is contraindicated in pregnant women and also is contraindicated in women contemplating pregnancy.
If itraconazole therapy for the treatment of onychomycosis is initiated in a woman of childbearing potential, the first dose of the drug should be given on the second or third day of the next normal menstrual period, and measures should be taken to ensure that effective contraception is continued throughout itraconazole therapy and for 2 additional months following discontinuance of the drug. In reproduction studies, itraconazole caused a dose-related increase in maternal toxicity, embryotoxicity, and teratogenicity in rats at dosages of approximately 40-160 mg/kg daily (5-20 times the maximum recommended human dosage) and in mice at dosages of approximately 80 mg/kg daily (10 times the maximum recommended human dosage).
Teratogenicity consisted of major skeletal defects in rats and encephaloceles and/or macroglossia in mice.
Although parental toxicity was observed, reproduction studies in male and female rats receiving oral itraconazole dosages up to 40 mg/kg daily (5 times the maximum recommended human dosage) did not reveal evidence of impaired fertility. More severe parental toxicity, including death, occurred at a dosage of 160 mg/kg daily (20 times the maximum recommended human dosage).
Itraconazole is distributed in human milk, and the expected benefits of itraconazole for the nursing woman should be weighed against the potential risk to the infant from exposure to the drug. Because of the potential for transmission of HIV to an uninfected child, the US Centers for Disease Control and Prevention (CDC) currently recommends that HIV-infected women not breastfeed infants.
Precautions Related to Cardiovascular Effects
Congestive heart failure, peripheral edema, and pulmonary edema have been reported in immunocompromised or immunocompetent patients receiving IV or oral itraconazole to treat systemic fungal infections and in immunocompetent patients receiving oral itraconazole capsules to treat onychomycosis. Itraconazole capsules should not be used to treat onychomycosis in patients with evidence of ventricular dysfunction, such as congestive heart failure, or a history of congestive heart failure.
For other indications (e.g., treatment of systemic fungal infections) in patients with evidence of ventricular dysfunction, IV or oral itraconazole should be used only when the benefits clearly outweigh the risks.
Clinicians should carefully review the risks and benefits of itraconazole therapy in patients with risk factors for congestive heart failure (e.g., those with cardiac disease such as ischemic and valvular disease, clinically important pulmonary disease such as chronic obstructive pulmonary disease, or renal failure and other edematous disorders), and IV itraconazole and itraconazole oral solution should be used with caution in these patients.
If itraconazole is considered necessary in patients with risk factors for congestive heart failure, they should be informed of the signs and symptoms of congestive heart failure and carefully monitored during therapy. Patients who develop congestive heart failure while receiving itraconazole oral capsules should discontinue the drug. If congestive heart failure occurs during therapy with IV itraconazole or itraconazole oral solution, the patient should be carefully monitored, and therapeutic options (including possible discontinuance of the drug) should be evaluated.
Because itraconazole and its major metabolite, hydroxyitraconazole, are potent inhibitors of the cytochrome P-450 (CYP) 3A4 isoenzyme system, concomitant use of the antifungal agent with drugs metabolized by these enzymes can increase plasma concentrations of these drugs resulting in potential increases in their therapeutic and adverse effects. Serious adverse cardiovascular effects (QT prolongation, torsades de pointes, ventricular tachycardia, cardiac arrest, and/or sudden death) have been reported in patients receiving itraconazole concomitantly with certain drugs metabolized by CYP3A4 enzymes (e.g., cisapride, dofetilide, pimozide, quinidine), and concomitant use with these drugs is contraindicated.
Precautions Related to Hepatic Effects
Because rare cases of serious hepatotoxicity have been reported with itraconazole, including some cases within the first week of therapy, itraconazole therapy should not be used in patients with increased serum hepatic enzymes, active liver disease, or a history of liver toxicity with other drugs unless the potential benefits exceed the risks. Serum hepatic enzyme concentrations should be monitored in any patient with preexisting hepatic function abnormalities and in those who have experienced liver toxicity with other drugs.
In addition, serum hepatic enzyme monitoring should be considered for all patients receiving itraconazole, especially those who receive itraconazole therapy continuously for longer than 1 month. Itraconazole should be discontinued immediately, and liver function testing should be performed if signs and symptoms consistent with liver disease develop during therapy. The risks and benefits of itraconazole should be reassessed in these patients. Patients should be instructed to stop itraconazole immediately and contact their clinician if any signs or symptoms of liver dysfunction occur, including unusual fatigue, dark urine, pale stool, anorexia, nausea, vomiting, or jaundice, so that appropriate laboratory testing can be performed.
Other Precautions and Contraindications
If neuropathy occurs that may be attributable to itraconazole, the drug should be discontinued. Because itraconazole may cause hypokalemia, some clinicians recommend monitoring serum potassium concentrations in patients receiving relatively high dosages and/or prolonged therapy with the drug. Itraconazole is contraindicated in patients with known hypersensitivity to the drug or any ingredient in the formulation.
Although information concerning cross-sensitivity between itraconazole and other triazole or imidazole antifungal agents is not available, the manufacturer states that itraconazole should be used with caution in individuals hypersensitive to other azoles.
Pediatric Precautions
The safety and efficacy of itraconazole in children younger than 18 have not been established. A limited number of patients aged 3-16 with systemic nonmeningeal fungal infections have received itraconazole capsules at a dosage of 100 mg daily without unusual adverse effects. In addition, a limited number of pediatric patients aged 6 months to 12 have received itraconazole oral solution at a dosage of 5 mg/kg once daily for 2 weeks without any unusual adverse effects.
However, data from animal studies have shown that itraconazole induces bone defects in rats receiving dosages as low as 20 mg/kg daily (2. times the maximum recommended human dosage). The defects included decreased bone plate activity, thinning of the zona compacta of large bones, and increased bone fragility. At a dosage of 80 mg/kg daily (10 times the maximum recommended human dosage) for longer than 1 year or 160 mg/kg daily (20 times the maximum recommended human dosage), the drug-induced small tooth pulp with a hypocellular appearance in some rats.
While bone toxicity observed in animals has not been reported to date in adult patients receiving the drug, the long-term effect of itraconazole therapy in children is not known.
Geriatric Precautions
Clinical studies of IV itraconazole did not include sufficient numbers of patients 65 years of age and older to determine whether geriatric patients respond differently than younger patients. While other clinical experience has not revealed differences in response, drug dosage should be selected cautiously in geriatric patients. The greater frequency of decreased hepatic, renal, and/or cardiac function and of concomitant disease and drug therapy observed in the elderly also should be considered.
Mutagenicity and Carcinogenicity
There was no evidence of mutagenicity when itraconazole was assayed in inappropriate bacterial, mammalian, and nonmammalian test systems. There was no evidence of carcinogenicity in mice receiving oral itraconazole for 23 months at dosages up to 80 mg/kg daily (about 10 times the maximum recommended human dosage). There was a slight increase in the incidence of soft tissue sarcoma in male rats receiving 25 mg/kg daily (3.1 times the maximum recommended human dosage). These sarcomas may have been a consequence of hypercholesterolemia, a response to chronic itraconazole administration observed in rats but not in dogs or humans.
An increase in the incidence of squamous cell carcinoma of the lung was observed in female rats receiving 50 mg/kg of itraconazole daily (6.25 times the maximum recommended human dosage). Although the occurrence of squamous cell carcinoma in the lung is extremely uncommon in untreated rats, the increased incidence in this study was not statistically significant. Commercially available itraconazole oral solution and itraconazole injection contain hydroxypropyl-b-cyclodextrin (HP-b-CD) as an excipient. HP-b-CD has produced pancreatic adenocarcinomas in rat carcinogenicity studies but similar effects were not observed in a mouse carcinogenicity study.
The clinical relevance of this finding is unknown. Based on body surface area comparisons, it has been estimated that patients receiving the usual dosage of commercially available itraconazole oral solution would be exposed to concentrations of HP-b-CD equivalent to 1.7 times the exposure of the lowest dose used in the rat study.
Whether findings in animal studies using orally administered HP-b-CD apply to parenterally administered itraconazole remains to be determined.
Other Features of the Patient
Adverse effects due to drug-drug interactions are not expected in diabetic patients using insulin and oral hypoglycemic drugs that are not metabolized by CYP3A4 (for example, tolbutamide, gliclazide, glibenclamide, glipizide, and metformin). The pharmacokinetic and safety data from clinical trials and postmarketing surveillance have been reviewed to assess the safety of itraconazole in diabetic patients with onychomycosis or dermatomycosis.
Postmarketing surveillance, including all adverse event reports in patients taking itraconazole concomitantly with insulin or an oral hypoglycemic drug, revealed 15 reports suggestive of hyperglycemia and nine reports suggestive of hypoglycemia. In most patients, the antidiabetic effect did not change. From clinical trials in 189 diabetic patients taking itraconazole for various infections, one itraconazole-related adverse event was recorded; this was aggravated diabetes in a renal transplant patient who was also taking ciclosporin.
Itraconazole has caused abnormalities in fetal development in rodents and is therefore contra-indicated in pregnancy.
Itraconazole should be avoided in patients with hepatic impairment. Liver function should be monitored if treatment lasts more than one month or if there are symptoms suggestive of hepatitis. Treatment should be stopped if abnormal liver function is detected.
Plasma-itraconazole concentrations should be monitored in patients with active liver disease, and the dosage adjusted if necessary. Dose adjustments may also be required in some patients with renal impairment. Licensed product information warns that the use of intravenous itraconazole preparations formulated with hydroxypropylbetadex is contraindicated in patients with a creatinine clearance of less than 30 mL/minute.
Itraconazole should be stopped if neuropathy develops. It should not be used to treat less severe fungal infections, such as onychomycosis, in patients with evidence of or a history of ventricular dysfunction, such as heart failure. Hypochlorhydria, which may be present in patients with AIDS, can reduce the absorption of itraconazole. In this case, absorption may be improved by giving itraconazole with an acidic drink, such as a cola beverage.
Preparations
| Dosage Form | Strength | Brand Name | Composition/Features | Manufacturer |
|---|---|---|---|---|
| Oral Capsules | 100 mg | Sporanox® | Available in PulsePak® and regular packages | Janssen |
| Oral Solution | 10 mg/mL | Sporanox® | Contains hydroxypropyl-β-cyclodextrin (400 mg/mL) and propylene glycol | Ortho Biotech |
| Parenteral for Injection | 10 mg/mL (250 mg concentrate for 200 mg IV delivery) | Sporanox® | Contains hydroxypropyl-β-cyclodextrin (400 mg/mL), propylene glycol, and 0.9% sodium chloride diluent; includes a filtered infusion set | Ortho Biotech |
Other Brand Names in Different Countries
| Country | Brand Names |
|---|---|
| Argentina | ITC, Itrac, Micotenk, Nitridazol, Panastat, Salimidin, Sporanox |
| Australia | Sporanox |
| Austria | Itrabene, Sporanox |
| Belgium | Sporanox |
| Brazil | Es-tiranox, Fungonax, Itracotan, Itrahexal-H, Itranax, Itraspor, Itrazol, Neo Itrax, Sporanox, Spozol, Tracnox, Traconal, Traconax, Tracozon, Trana-zol, Tratzol, Traxonol |
| Canada | Sporanox |
| Chile | Itodal, Sporanox, Teramic |
| Czech Republic | Cladostad, Prokanazol, Sporanox |
| Denmark | Niddazol, Sporanox |
| Finland | Sporanox |
| France | Sporanox |
| Germany | Canifug, Itra, Itracol, Sempera, Siros |
| Greece | Assosept-S, Bevonazole, Brovicton, Deratil, Etrel, Flunol, Idranox, Isoflon, Itrabest, Itracon, Itraconal, Itralfa, Itraviron, Itrazol, Laverio, Lorenzol, Mesmor, Micronazol, Prominox, Soprazon, Sporanox, Sporizole, Stas, Sterginox, Zetilox |
| Hong Kong | Aranox, Quali-itrazole, Sporanox |
| Hungary | Cladostad, Omicral, Orungal |
| India | Candistat, Canditral, Itracan |
| Indonesia | Forcanox, Fungitrazol, Furolnok, Itzol, Nufatrac, Sporacid, Sporanox, Spyrocon, Trachon, Unitrac, Zitrazol |
| Ireland | Sporanox |
| Israel | Itranol, Sporanox |
| Italy | Sporanox, Trazer, Triasporin |
| Malaysia | Canditral, Inox, Sporanox, Unitrac |
| Mexico | Carexan, Conamed, Congox, Derusil, Ergospharma, Fitocyd, Fulzoltec, Fuzoltec, Imazol, Iqcona, Isoporum, Isox, Itranax, Lozartil, Rixtal, Seritral, Silicsan, Sinozol, Solmavin, Sporanox, Steitraz, Trax, Z-Fin, Zolken |
| The Netherlands | Trisporal |
| Norway | Sporanox |
| New Zealand | Sporanox |
| Philippines | Sporanox |
| Poland | Orungal, Trioxal |
| Portugal | Sporanox |
| Russia | Irunine, Orungal, Orungamin, Orunit, Rumycoz |
| South Africa | Adco-Sporozole, Sporanox |
| Singapore | Canditral, Sporanox |
| Spain | Canadiol, Hongoseril, Oromic, Sporanox |
| Sweden | Sporanox |
| Switzerland | Sporanox |
| Thailand | Canditral, Icona, Itra, Itracon, Norspor, Spazol, Sporal, Sporlab, Spornar |
| Turkey | Funit, Itraspor, Sporex |
| United Kingdom | Sporanox |
| United States | Sporanox |
| Venezuela | Fungosin, Sporanox |
Multi-ingredient
- Mexico: Sepia Sporasec
- Venezuela: Sporasec


 (37 votes, average: 4.03 out of 5)
(37 votes, average: 4.03 out of 5)















