Essentials of Diagnosis
- Intranuclear inclusions and multinucleated giant cells in tissue cytology.
- Grows rapidly in many types of tissue culture.
- HSV antigen can be detected in tissue by immunofluorescence.
- Polymerase Chain Reaction (PCR) analysis of cerebrospinal fluid (CSF) now considered best assay for HSV encephalitis.
General Considerations
Epidemiology
The term herpes (from the Greek herpein, to creep) and the clinical description of cold sores date back to Hippocrates. Two distinct epidemiologic and antigenic types of HSVs exist (HSV-1 and HSV-2). HSVs have worldwide distribution. There are no known animal vectors, and humans appear to be the only natural reservoir (Box 33-1 shows the syndromes caused by HSV). Direct contact with infected secretions is the principal mode of spread. Seroepidemiologic studies indicate that the prevalence of HSV antibody varies directly with the age and socioeconomic status of the population studied. In most underdeveloped countries, 90% of the population have HSV-1 antibody by the age of 30. In the United States, HSV-1 antibody is currently found in ~ 50-60% of the middle-class population. Among lower socioeconomic groups, however, the percentage approaches 90%.
Detection of HSV-2 antibody before puberty is unusual. The virus is associated with sexual activity, and direct sexual transmission is the major mode of spread. Approximately 15-30% of sexually active adults in western industrialized countries have HSV-2 antibody. The virus can be isolated from the cervix and urethra of ~ 5-12% of adults attending sexually transmitted disease clinics; many of these patients are asymptomatic or have small, unnoticed lesions on penile or vulvar skin. Genital herpes is not a reportable disease in the United States, but it is estimated that 500,000 new cases occur per year.
Microbiology
The DNA genomes of both types of HSV are linear, double-stranded molecules with molecular weights of ~ 108 and are composed of ~ 160 kilobase pairs. Their nucleic acids demonstrate ~ 50% base sequence homology, which is considerably greater than that shown between these viruses and other herpesviruses. HSV-1 and HSV-2 share antigens in almost all their surface glycoproteins and other structural polypeptides. Numerous strains of both HSV-1 and HSV-2 exist. In fact, by restriction endonuclease analysis of the viral genome, most isolates of HSV-1 or HSV-2 are found to differ somewhat, except in epidemiologically related cases such as mother-infant and sexual partner transfer.
Pathogenesis
Herpes simplex virus produces both acute and latent infections.
1. Acute Infection. In acute infections, the initial stages entail envelope glycoprotein-mediated attachment of the virus to unidentified receptors and fusion with the host cell membrane. Viral DNA released in the cytoplasm is transported through nuclear pores to the nucleus. New viral DNA synthesis and transcription of mRNA occur in the nucleus. The virus buds through the nuclear membrane; this process adds the envelope material to the virus particles, which are then transported through the cytoplasm and out of the cell in a manner similar to the movement of other proteins.
The molecular events involving synthesis of virus-specific gene products are coordinated and regulated. Three classes of mRNA coding for three groups of virus polypeptides have been identified. The products, designated the alpha or immediate early (IE) polypeptides, are synthesized 2-4 h after infection. These IE polypeptides probably function as regulators of viral transcription. The beta or early (E) polypeptides include virus-specified thymidine kinase (TK) and DNA polymerase (Pol). These virus-specified enzymes differ from host cell enzymes and are therefore important targets of antiviral chemotherapy. The synthesis of early polypeptides shuts off the synthesis of immediate early polypeptides and induces the synthesis of a third group of gamma or late (L) polypeptides. The late polypeptides, synthesized 12-15 h after infection, are the major structural components of the viral particle.
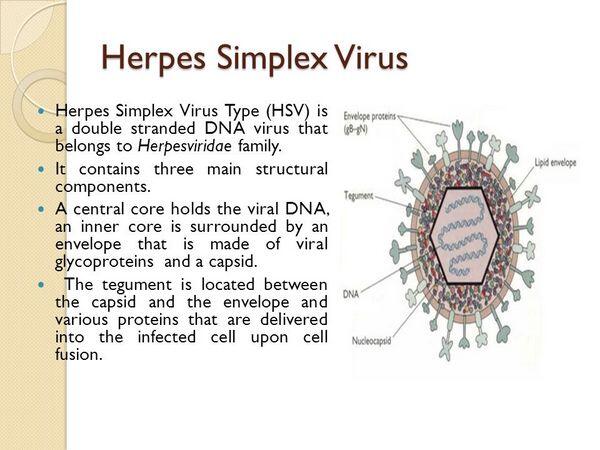
Pathologic changes during acute infections consist of development of multinucleated giant cells, ballooning degeneration of epithelial cells, focal necrosis, eosinophilic intranuclear inclusion bodies, and an inflammatory response characterized by an initial polymorphonuclear neutrophil infiltrate and a subsequent mononuclear cell infiltrate. The virus can spread intra- or interneuronally or through the supporting cellular networks of an axon or nerve, resulting in latent infection of sensory and autonomic nerve ganglia. The spread of virus can occur by cell-to-cell transfer and can therefore be unaffected by circulating immune globulin.
2. Latent Infection. In humans, latent infection by HSV-1 has been demonstrated, by co-cultivation techniques, in trigeminal, superior cervical, and vagal nerve ganglia and occasionally in the S2-3 dorsal sensory nerve root ganglia. Latent HSV-2 infection has been demonstrated in the sacral (S2-3) ganglia. Latent infection of neural tissue by HSV does not result in the death of the cell; however, the exact mechanism of viral genome latency is incompletely understood. The HSV genome exists in a circular form in latently infected neuronal cells. Transcription of only a small portion of the viral genome is abortive and does not appear to be associated with detectable amounts of early eg, (Pol) or TK, or late polypeptides. Therefore, unfortunately, antiviral drugs directed at the viral DNA polymerase do not eradicate the virus in its latent state.
Reactivation of virus from latently infected ganglionic cells with subsequent release of infectious virions appears to account for most recurrences of both genital and oralabial infections. The mechanisms by which latent infection is reactivated are unknown. Precipitating factors that initiate reactivation of herpes simplex include fever, trauma (eg, oral intubation), and exposure to ultraviolet light.
Immunity
Host factors have a major effect on clinical manifestations of HSV infection. Many episodes of HSV infection are either asymptomatic or mildly symptomatic. If initial clinical episodes of the disease are symptomatic, they are more severe than recurrent episodes, probably because of the presence of anti-HSV antibodies and immune lymphocytes in persons with recurrent infections.
Neutralizing antibodies directed against HSV envelope glycoproteins appear to be important protective responses, particularly those that mediate antibody-dependent cellular cytotoxicity (ADCC) reactions. ADCC may be important in limiting early spread of HSV. By the second week of infection, cytotoxic T lymphocytes can be detected that are able to destroy HSV-infected cells before completion of the replication cycle. Prior infection with HSV-1 may protect against or shorten the duration of symptoms and lesions during subsequent infection with HSV-2. In immunosuppressed patients, especially those with depressed cell-mediated immunity, reactivation of HSV may be associated with prolonged viral excretion and persistence of lesions.
CLINICAL SYNDROMES
HERPES SIMPLEX TYPE 1 (HSV-1)
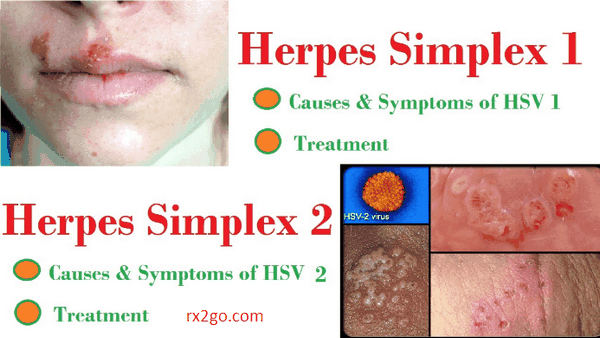
Clinical manifestations of infection with HSV-1 usually are found above the waist. They consist characteristically of grouped or single vesicular lesions that become pustular and coalesce to form single or multiple ulcers. On dry surfaces, these ulcers scab before healing; on mucosal surfaces, they re-epithelialize directly. HSV can be isolated from almost all ulcerative lesions, but the titer of virus decreases as the lesions progress. Infections generally involve ectoderm (skin, mouth, vagina, conjunctiva, and nervous system).
Primary infection with HSV-1 is often asymptomatic. When clinically evident, it appears most frequently as gingivostomatitis with fever, and vesicular or ulcerative lesions involving the buccal mucosa, tongue, gums, and pharynx. The lesions are quite painful, and the illness usually lasts 5-12 days. After this initial infection, HSV may become latent within sensory nerve root ganglia of the trigeminal nerve.
Recurrent lesions usually appear on an area of the lip and the immediately adjacent skin; these lesions are described as mucocutaneous and are commonly called cold sores or fever blisters. Because reactivation is usually from a single latent source, these lesions are typically unilateral. Their recurrence may be signaled by premonitory tingling or burning in the area. Systemic complaints are unusual, and the episode generally lasts approximately 7 days. HSV may be reactivated and excreted into the saliva with no apparent mucosal lesions present. HSV has been isolated from saliva in 5-8% of children and 1-2% of adults who were asymptomatic at the time.
HSV sometimes infects the finger or nail area. This infection, termed herpetic Whitlow, usually results from the inoculation of infected secretions through a small cut in the skin. Painful vesicular lesions of the finger develop and pustulate and are often mistaken for bacterial infection and mistreated accordingly. Health care workers, especially nurses and respiratory therapists, are at particular risk for this entity.
HSV infection of the eye is one of the most common causes of corneal damage and blindness in the developed world. Infections usually involve the conjunctiva and cornea, and characteristic dendritic ulcerations are produced. With recurrence of disease, there may be deeper involvement with corneal scarring. Occasionally there may be extension into deeper structures of the eye, egiritis, especially if topical steroids are used.
HSV encephalitis is a rare result of HSV-1 infection, occurring in ~ 1-10 humans/million/year. Although rare, herpes encephalitis accounts for ~ 10% of all cases of documented viral encephalitis in the United States. Most cases occur in adults with high levels of anti-HSV-1 antibody, suggesting reactivation of latent virus in the trigeminal nerve root ganglion and extension of productive (lytic) infection into the temporoparietal area of the brain.
Since the disease usually affects one temporal lobe, focal neurologic signs are frequent. Clinically, the disease can resemble brain abscess, tumor, or intracerebral hemorrhage.
HERPES SIMPLEX TYPE 2 (HSV-2)
Genital herpes is a common sexually transmitted disease. Both HSV-1 and HSV-2 can cause genital disease, and the symptoms and signs of acute infection are similar for both viruses. Of first episodes of genital HSV infection in the United States, 70% are caused by HSV-2. As with type 1 oral infection, the majority of genital infections are asymptomatic without lesions, and patients do not know they have been infected. Patients with asymptomatic genital HSV may be culture or PCR positive in genital secretions and are able to transmit the virus to sex partners.
Primary Genital Herpes Infection
The mean incubation period from sexual contact to onset of lesions is 5 days. Lesions begin as small erythematous papules that soon form vesicles and then pustules. Within 3 to 5 days the vesiculopustular lesions break to form painful coalesced ulcers that subsequently dry; some form crusts and heal without scarring. With primary disease the genital lesions are usually multiple, bilateral, and extensive. The urethra and cervix are also infected frequently, with discrete or coalesced ulcers on the exocervix. Bilateral enlarged tender inguinal lymph nodes are usually present and may persist for weeks to months. About one-third of patients show systemic symptoms such as fever, malaise, and myalgia, and 1-10% develop aseptic meningitis with neck rigidity and severe headache. First episodes of disease usually last 20-30 days.
Recurrent Genital Infection
In contrast to primary infection, recurrent genital herpes is a disease of shorter duration, usually localized in the genital region, without systemic symptoms. Prodromal paresthesias in the perineum, genitalia, or buttocks occur 12-24 h before the appearance of lesions. Recurrent genital herpes usually presents with grouped vesicular lesions in the external genital region. Local symptoms such as pain and itching are mild, lasting 4-5 days, and lesions usually last 10-14 days. Recurrent meningitis due to HSV-2 does occur.
NEONATAL HERPES
Neonatal herpes usually results from transmission of virus during delivery, as the neonate passes through infected genital secretions of the mother. True congenital in utero infection, although possible, is uncommon. The prevalence rate of neonatal herpes varies greatly among populations but is estimated at ~ 1/2500 live births in the United States. This estimate is based on the observation that ~ 0.5-1.0% of women excrete HSV from the cervix at the onset of labor and ~ 6% of babies born through infected birth canals develop neonatal HSV.
Manifestations of neonatal herpes vary. To a considerable degree, this is determined by the mother’s antibody status. If she is experiencing primary HSV infection and has no antibody to pass to the baby, the consequences can be severe. If she is experiencing a reactivation, the baby can be completely protected by maternal antibody. Some infants show disseminated skin lesions only; others have widespread internal organ involvement; still others have involvement of the central nervous system only, with listlessness and seizures. Less commonly, HSV-1 causes neonatal herpes infection, usually resulting from genital HSV-1 lesions or colonization.
Diagnosis
Herpes simplex virus is easily isolated from lesions or tissue by using fibroblasts or a variety of other tissue culture cells. The cytopathic effects of HSV can usually be demonstrated 24-48 h after inoculation. Isolates of HSV-1 and HSV-2 (see below) can be differentiated by staining virus-infected cells with type-specific monoclonal antibodies to the two types or by analyzing restriction enzyme digests of purified viral DNA. Restriction endonuclease digests can also be used to define epidemiologic relationships, that is, identify strains acquired between sexual partners or through mother-infant transmission. A direct smear prepared from the base of a suspected lesion and stained by either the Giemsa or Papanicolaou method may show intranuclear inclusions or multinucleated giant cells typical of herpes (as demonstrated by the Tzanck test) but is less sensitive than a viral culture and doesn’t distinguish HSV-1 from HSV-2. Enzyme immunoassays have been developed for direct detection of herpes antigen in lesions. Although early versions of these noncultural tests lacked sensitivity, more recent assessments show ~ 90% correlation with results from cultures. Rapid diagnosis of HSV mucocutaneous lesions can be accomplished with immunofluorescence or other antigen detection methods. Serology should not be used to diagnose active HSV infection, for example, genital or encephalitis; frequently there is no change in antibody titer when reactivation occurs. PCR on CSF is now the test of choice to diagnose HSV encephalitis. PCR positivity eliminates the need for brain biopsy, but the latter should still be performed if PCR is negative in a patient with highly suggestive clinical findings.
Treatment
Several antiviral drugs have been developed that inhibit HSV (Box 33-2). The most commonly used is the nucleoside analog acyclovir (INN: Aciclovir), which is converted by a viral enzyme (thymidine kinase) to a monophosphate and then by cellular enzymes to the triphosphate form, which is a potent inhibitor of the viral DNA polymerase. Acyclovir significantly decreases the duration of primary infection but has much less effect when used for treating recurrent infection. Valacyclovir is a prodrug of acyclovir that is better absorbed and can be used in lower and less frequent doses. Famciclovir is an oral drug that is converted to penciclovir, has good bioavailability, is equivalent to acyclovir but can also be given less frequently.
Prognosis
At least 80% of patients with clinically evident primary HSV infection develop recurrent episodes of herpes within 12 months. In patients whose lesions recur, the median number of recurrences is four or five per year. They are not evenly spaced, and some patients experience a succession of monthly attacks followed by a period of quiescence. Most recurrences result from reactivation of virus from dorsal root ganglia. Rarely, recurrent diseases may be caused by reinfection with a different strain of HSV. Ultimately recurrences of herpes diminish in frequency, especially with genital HSV-2 infection. Genital HSV-1 infection recurs to recur less frequently.
If untreated, HSV-1 encephalitis has a mortality of 70%, but intravenous acyclovir reduces mortality especially if given before coma occurs.
Because a normal immune response is absent in the neonate, neonatal HSV infection is an extremely severe disease with an overall mortality of ~ 60%, and neurologic sequelae are high in those who survive.
Prevention & Control
Avoiding contact with individuals with lesions reduces the risk of spread; however, virus may still be spread by individuals shedding virus asymptomatically from the saliva, urethra, and cervix. When lesions are present, sexual intercourse should be avoided. Condoms should be used when individuals with a history of type 2 HSV or antibodies have sexual contact with susceptible people, even when lesions are absent. Daily acyclovir, valacyclovir, or famciclovir suppresses recurrences of HSV infections and is indicated for patients who have frequent attacks (Box 33-3).
Because of the high morbidity and mortality of neonatal infection, special attention must be paid to preventing transmission during delivery. In some cases Cesarean section may be used to minimize contact of the infant with infected maternal genital lesions; however, Cesarean section may not be effective if rupture of the membranes precedes delivery by several hours. A recently completed trial of a recombinant HSV-2 vaccine in HSV-2-infected patients had no impact on the rate of recurrences. It is not known whether a vaccine would prevent primary infection, although an effective vaccine offers the best hope for controlling the spread of genital herpes infections. Several different vaccines are at various stages of development and testing. Subsequently a recombinant HSV vaccine was similarly ineffective in preventing primary genital HSV-2 infection.


 (1 votes, average: 4.00 out of 5)
(1 votes, average: 4.00 out of 5)


I want to share my story. I contracted herpes as a teenager. It literally controlled my life. As I grew older, it intensified with outbreaks. I’m 48 and I can say that I’m in remission. I’m a health and fitness professional and have done a tremendous amount of research, especially on herpes. A year ago, I came across Dr Oba Herbs.I contacted Dr Oba and he requested for my personal information and i sent them to him,Dr Oba prepared the liquid medicine and sent to me via DHL delivery service and i took the medicine 3 times daily for 14days… Read more »